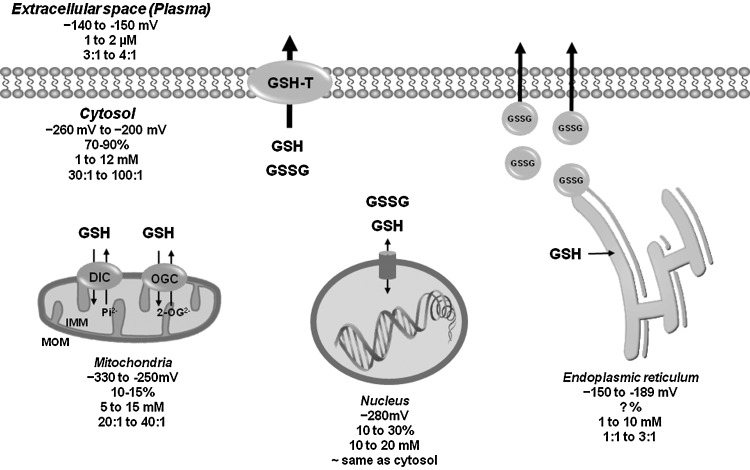
FIG. 3.
Compartmentalization of the GSH/GSSG redox couple. GSH is produced intracellularly and is found 70%–90% freely distributed in the cytosol, but also compartmentalized in mitochondria, nuclear matrix, and ER. Specific transport mechanisms maintain compartmentalized GSH/GSSG homeostasis. GSH diffuses through MOM via porin channels (not depicted here), and translocates through the IMM via DIC or OGC exchangers. In the nucleus, GSH is considered to diffuse freely through the nuclear pore. Protein-dependent facilitated diffusion is thought to mediate GSH permeation in the ER, but the molecular identify of the mechanism(s) involved remains unknown. Within the ER, GSH exits largely as GSSG due to its oxidation. It has been proposed that GSSG could be secreted via the secretory pathway for its recycle. A variety of protein transporters have been reported to act as plasma membrane GSH transporters (GSH-T), but their role in GSH depletion during cell death progression is still unclear. Values indicate redox potential for GSH/GSSG (mV), % of compartmentalized GSH with respect to total cellular levels, concentration of GSH (mM), and GSH/GSSG ratio for each subcellular compartment. Values were taken from (6, 95, 128, 212). IMM, inner mitochondrial membrane; DIC, dicarboxylate carrier; OGC, 2-oxoglutarate transporters.