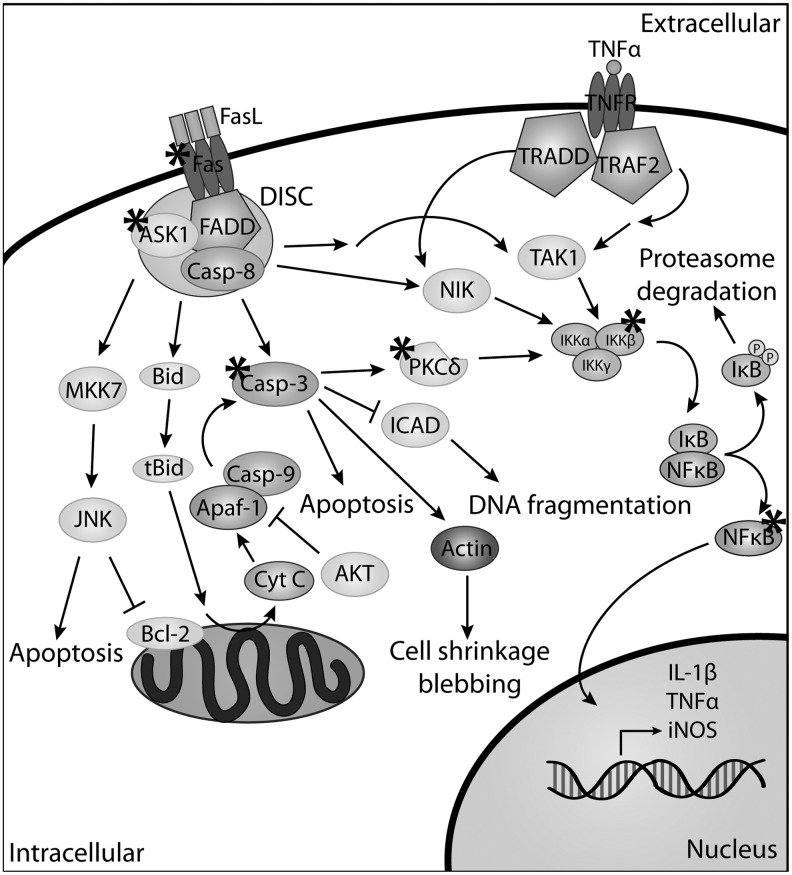
FIG. 3.
Overview of apoptotic signaling through tumor necrosis factor superfamily receptor 6 (Fas) and tumor necrosis factor receptor (TNFR). When Fas ligand (FasL) (CD95L) binds to Fas it initiates the formation of the death-inducing signaling complex (DISC) involving the association of Fas-associated death domain (FADD) and pro-caspase-8 (pro-casp-8). In the absence of caspase-8-FADD-like interleukin-1β-converting enzyme inhibitory protein (c-FLIP), pro-caspase-8 is activated and initiates the caspase proteolytic cascade, leading to apoptosis. DISC formation can also lead to the activation of the MKK7 pathway and jun N-terminal kinase (JNK) phosphorylation, resulting in apoptosis. There are other cell-type-specific pathways that are affected by FasL. One of these pathways involves Bid, which can become truncated Bid (tBID) leading to the release of cytochrome C, and subsequently caspase activation. FasL can also stimulate the IκB kinase (IKK)/NFκB pathway resulting in the phosphorylation of IκB, leading to proteasome degradation and the translocation of NFκB to the nucleus. NFκB is responsible for initiating transcription of proteins, such as Grx, iNOS, and TNFα. The NFκB pathway can also be activated through the binding of TNFα to the TNFR, acting through NIK or TAK1. Proteins in these pathways for which there is experimental evidence implicating that they are regulated by Grx and protein glutathionylation are indicated by an asterisk (*).