Fig 4.
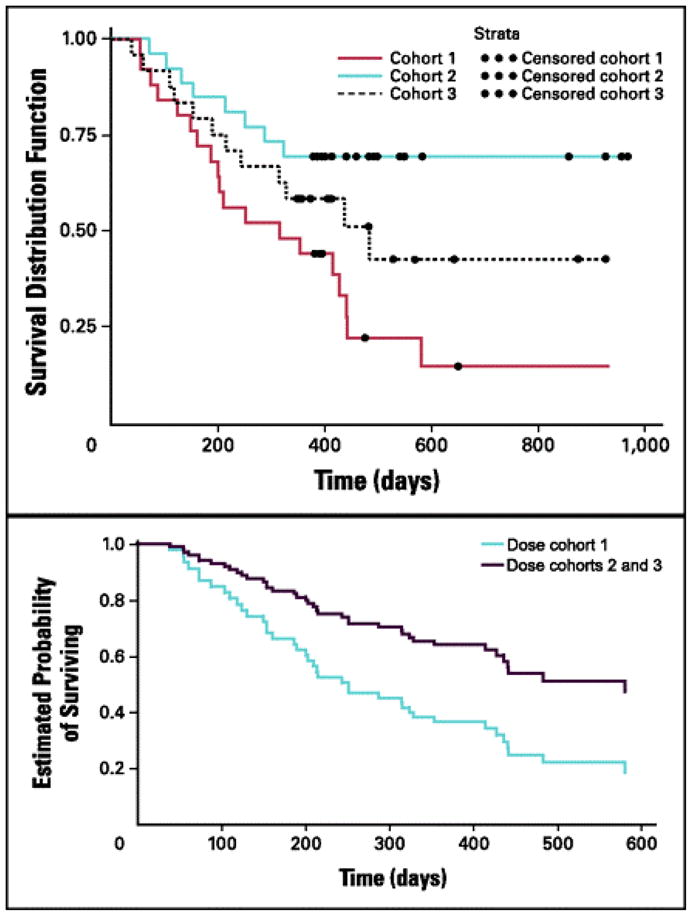
Phase II study of belagenpumatucel-L, a TGF-β2 antisense gene-modified allogeneic tumor cell vaccine in non-small cell lung cancer. Panel A: Dose-related survival between cohorts (1.25, 2.5,or 5.0 × 107 cells/injection) for all patients (N = 75; P = .0155). Panel B: Overall survival for cohort 1 versus cohorts 2 and 3 for advanced-stage patients (n = 61; P = .0186).
Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Nemunaitis J, et al. J Clin Oncol 2006;24:4721–30.
