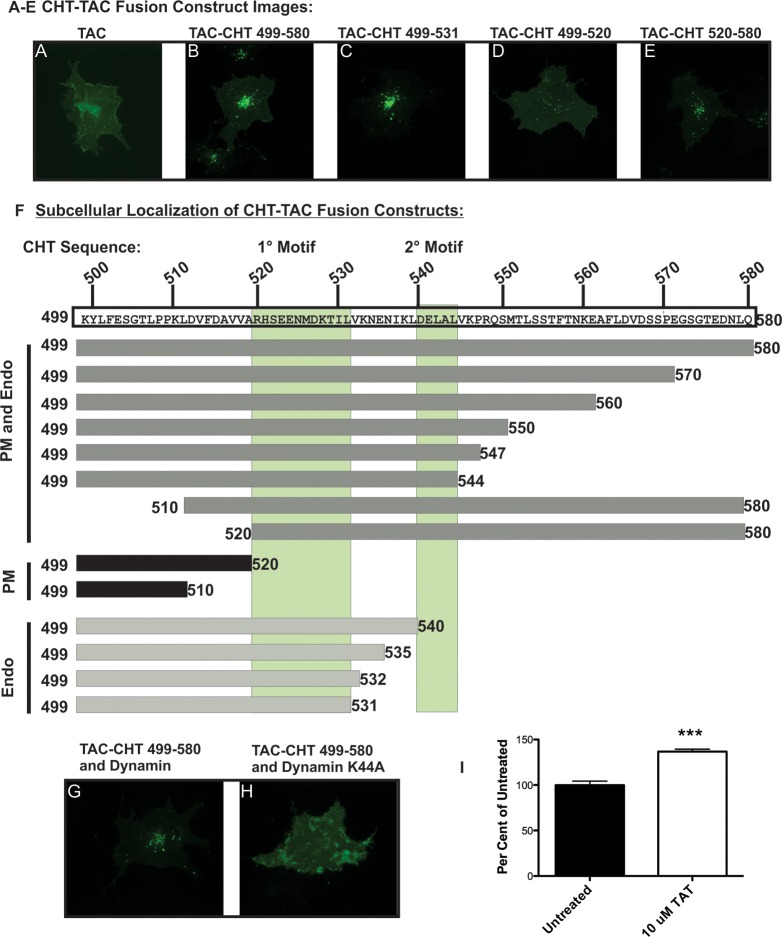
Figure 1.
TAC–CHT fusion proteins indicate a discrete motif regulating CHT trafficking resides in the C-terminus. (A) Surface labeled Tac remains concentrated at the plasma membrane in HEK293 cells. (B) The full length CHT C-terminus directs internalization and sorting of the Tac-CHT chimera to a perinuclear endocytic compartment. (C) CHT amino acids 499–531 direct endocytosis when fused to Tac. This construct exhibits enhanced internalization compared to the full length CHT C-terminus. (D) Stable localization at the plasma membrane indicates that CHT amino acids 499–520 do not support efficient endocytic sorting of a Tac chimera. (E) Endocytic sorting of the Tac-CHT persists following deletion of CHT amino acids 498–519 (Tac-CHT520–580). (F) A summary of the localization of Tac-CHT deletion mutants presented in this figure points to CHT amino acids 522–532 as a 1° endocytic motif. A 2° motif appears between CHT amino acids 540–544 that facilitates CHT plasma membrane localization. (G) Cotransfection of WT dynamin I does not impair the endocytic sorting of Tac-CHT (Scale bar = 20 μm). (H) Expression of the dominant negative K44A mutant of dynamin I blocks internalization of the Tac-CHT chimera. (I) Saturation of the endocytic machinery by the CHT endocytic motif is seen as a 40% increase in CHT choline uptake activity following treatment of CHT expressing HEK293 cells with 10 μM of a CHT-TAT peptide fused to amino acids 524–539.