Abstract
Previous experiments have assessed planning during sequential responding to computer generated stimuli by Old World nonhuman primates including chimpanzees and rhesus macaques. However, no such assessment has been made with a New World primate species. Capuchin monkeys (Cebus apella) are an interesting test case for assessing the distribution of cognitive processes in the order Primates because they sometimes show proficiency in tasks also mastered by apes and Old World monkeys, but in other cases fail to match the proficiency of those other species. In two experiments, eight capuchin monkeys selected five arbitrary stimuli in distinct locations on a computer monitor in a learned sequence. In Experiment 1, shift trials occurred in which the second and third stimuli were transposed when the first stimulus was selected by the animal. In Experiment 2, mask trials occurred in which all remaining stimuli were masked after the monkey selected the first stimulus. Monkeys made more mistakes on trials in which the locations of the second and third stimuli were interchanged than on trials in which locations were not interchanged, suggesting they had already planned to select a location that no longer contained the correct stimulus. When mask trials occurred, monkeys performed at levels significantly better than chance, but their performance exceeded chance levels only for the first and the second selections on a trial. These data indicate that capuchin monkeys performed very similarly to chimpanzees and rhesus monkeys and appeared to plan their selection sequences during the computerized task, but only to a limited degree.
Keywords: Planning, Capuchin monkeys, Cebus apella, Computer testing, Sequence learning
Introduction
Planning requires the organization of behavior in the present to obtain a future goal, and this goal can occur seconds, hours, days, or years in the future (Miller, Galanter and Pribram 1986). Although many of the most impressive feats of planning involve long time intervals between the organization and implementation (e.g., planting seeds to harvest crops months later, or saving money for retirement), planning also is important in situations in which multiple actions must be completed in a specific sequence in order to achieve a goal or to complete a task in the more immediate future (e.g., preparing various food items in the order they are needed to complete a recipe). These abilities can be easily tested across a variety of nonhuman species in an attempt to illustrate whether and how other species perform when actions must be organized and completed in pursuit of some goal.
Tests of temporally limited sequence planning have been given to a number of species, and in many cases using nearly identical methodologies. For example, various New World and Old World monkey species may plan travel routes through computerized mazes (Fragaszy, Johnson-Pynn, Hirsh and Brakke 2003; Fragaszy, Kennedy, Murnane, Menzel, Brewer, Johnson-Pynn and Hopkins 2009; Mushiake, Saito, Sakamoto, Sato and Tanji 2001; Washburn 1992). Another line of research into animal planning required animals to learn to sequence visual stimuli in a particular order (e.g., D’Amato and Colombo 1988, 1989; Terrace 1986). Biro and Matsuzawa (1999) and Kawai and Matsuzawa (2000) reported that a chimpanzee named Ai performed a sequencing task on a touch screen computer monitor. Ai made ordered selections of Arabic numerals. Sometimes the selection of the first number was followed by the covering of all other numbers, typically referred to as a mask condition. When that occurred, Ai could only succeed if she had already planned the remainder of the sequence, or at least remembered where all of the other numerals originally had been located so that she could select them even after they were covered (Kawai and Matsuzawa 2000). Ai was successful on those trials, selecting the four remaining numerals even after they were covered, and subsequent studies showed similar results with even larger numbers of stimuli (e.g., 9 items by Inoue and Matsuzawa, 2007).
A more convincing demonstration that Ai planned her sequences came from the trials in which stimuli locations were interchanged, typically referred to as a shift condition (Biro and Matsuzawa 1999). Ai was significantly more likely to make an error on these trials than on baseline trials in which the locations of the second and third numbers were not interchanged. One explanation for this result was that she had already planned to move to the second location even before the stimuli were transposed, and so the shift caused her to make an error due to implementation of that intended next response.
Beran, Pate, Washburn, and Rumbaugh (2004) gave these same tests to other chimpanzees and to rhesus monkeys, but with only limited success in replicating the results shown by Ai. All of the animals in that study demonstrated errors when the locations of items were shifted, suggesting that they were planning beyond the current response to at least the next one. However, in the mask condition, these chimpanzees and monkeys only performed above chance for one additional item (selection number two in the sequence) after the masks were presented. So, there was no evidence that they planned the entire selection sequence, but instead appeared to be looking only one move ahead.
To date, only apes and Old World monkey species have been given this test among primate species, with no data coming from studies with New World primate species. However, pigeons provided evidence that non-primate species could show similar performance to some of the primates. Pigeons were trained to respond to three stimuli in a learned order, then experienced the same probe trials (shift and mask trials) as those used with primates (Scarf and Colombo 2010). As with the primates tested by Beran et al. (2004), planning was limited among the pigeons, but still evident, as only one masked item was selected at above chance levels, and the shift trials also had the same detrimental effects seen in primates (e.g., Beran et al. 2004; Biro and Matsuzawa 1999). In addition, Scarf, Danly, Morgan, Colombo, and Terrace (2011) reported that shifts of the stimuli in positions three and four in the sequence after choice of the first stimulus had little or no effect on performance of monkeys, further indicating that they were not planning the entire sequence of responses at the outset of trials. Thus, one might assume that these results suggest similarities across a broad range of species for this type of planning behavior, with evidence of only limited planning in a sequence task. But, there is good reason to assess the performance of yet other species, including a New World primate species. Planning, even of the limited form seen in some species using these computerized tests, could be considered an “executive function” that is reliant on higher-order cognitive processes including executive attention and cognitive control. These functions are not ubiquitous among primates, at least in their depth of demonstration, and so there could be reason to expect species differences even though most comparative analyses to date have shown relatively consistent success levels for planning tasks like those already outlined.
Capuchin monkeys are an interesting test case. In many respects, they are considered the “poor person’s chimpanzee” with cognitive skills closely in line with those shown by apes and Old World monkey species in a variety of tasks including, but not limited to, tests of quantity judgment, self control, tool use, concept formation, analogical reasoning, and spatial representations (Beran 2008; Beran et al. 2008a, 2008b; D’Amato and Colombo 1988; Evans, Beran and Addessi 2010; Evans and Westergaard 2004; Flemming 2011; Judge, Evans and Vyas 2005; Kennedy and Fragaszy 2008; McGonigle, Chalmers and Dickinson 2003; Poti et al. 2010; Wright and Katz 2006; Yocum and Boysen 2010). But, recent research has indicated that capuchin monkeys lack one capacity that both apes and Old World monkeys (and perhaps even other species) have been argued to possess: metacognition. Chimpanzees and rhesus monkeys succeed in tasks requiring memory monitoring and respond flexibly to uncertainty by avoiding responding on trials for which difficulty is high, potentially providing evidence of metacognition or at least the fundamental foundations of a higher-order metacognitive system (e.g., Beran and Smith 2011; Call, 2012; Call and Carpenter 2001; Hampton 2001; Kornell, Son and Terrace 2007; Smith, Beran, Redford and Washburn 2006; Suda-King 2008). In contrast to this, capuchin monkeys fail in similar tasks and do not use optimal information-seeking strategies or indicate uncertainty in other tests where information must be selectively obtained (Basile, Hampton, Suomi and Murray 2009; Beran and Smith 2011; Beran, Smith, Coutinho, Couchman and Boomer 2009; Fujita 2009; Paukner, Anderson and Fujita 2006). At present, it remains unclear whether other species show such metacognitive capacities, with evidence for and against such abilities in diverse species such as rats (Foote and Crystal 2007, 2012) and pigeons (Adams and Santi 2011; Inman and Shettleworth 1999; Sutton and Shettleworth 2008). Thus, the capuchin monkey stands out as an interesting “failure” in this area of research, and may yield exciting insights into the relationship between metacognition and planning. Additionally, further investigating the areas in which the capuchin monkey differs from Old World primate species is illuminating to determine whether these differences are one of degree or kind (Darwin, 1871).
There are a number of ways in which capacities for planning and for metacognition should overlap where other cognitive abilities (such as numerical judgment or tool use) do not. Both processes rely on active monitoring (e.g., Flavell 1979; Nelson and Narens 1994), presumably through the use of general executive functions including attention allocation and cognitive control, and likely are supported by the same cortical areas (Ardilla 2008). Some have argued that the planning of responses with concurrent monitoring of informational states in relation to those planned responses is one example of metacognitive executive functioning (see Ardilla, 2008). Given this proposed link between high-level cognitive capacities such as planning and metacognition, it is an open question as to how capuchin monkeys would perform on planning tasks like those given to chimpanzees and Old World monkeys. Do capuchin monkeys show planning abilities like those of other primates, or are they lacking in this capacity relative to other primate species? If they are lacking, this would perhaps illustrate through new behavioral evidence some link between the ability to plan future responses and the capacity for metacognitive monitoring. However, if capuchin monkeys looked like other primates in their planning capacity, it would further highlight their shared capacities, including for an executive functioning system in some ways like that of Old World monkeys and apes. This would show that capuchin monkeys can access certain executive processes despite their apparent lack of metacognitive ability. In two experiments, we presented capuchin monkeys with the exact same test given to rhesus monkeys and chimpanzees by Beran et al. (2004) in an attempt to answer these questions and provide new insights into the planning and sequencing abilities of nonhuman primates.
Experiment 1
In the first experiment, the shift procedure was introduced to the monkeys. First, the monkeys selected a set of arbitrary stimuli in a learned sequence using a joystick-controlled computer task. After learning the sequence of stimuli in these baseline trials, we introduced infrequent probe trials in which two stimuli on the screen shifted locations after a monkey correctly selected the first stimulus in the sequence. The two stimuli that shifted were in positions two and three of the sequence, and so the next item to be selected would have moved locations after the first selection. If monkeys were planning their selections, they should have made more errors during shift trials than during baseline trials because the monkeys should have already determined what selections they intended to make for at least the next selection (if not the whole trial). Additionally, even if monkeys observed the interchange of stimuli but were implementing a sequence of planned responses, their response times should have been longer on shift trials than on trials without shifts. Longer response times in shift trials were expected because the monkeys would have to change the joystick movement from the original location they had planned to select (a decision made prior to the shift) to where the correct stimulus was located (a decision made after the shift).
Method
Participants
We tested eight capuchin (Cebus apella) monkeys: Logan (age 6 male), Liam (age 7 male), Nala (age 8 female), Wren (age 8 female), Lily (age 14 female), Griffin (age 14 male), Gambit (age 15 female), and Drella (age 21 male). Capuchin monkeys were group housed but separated for testing. All monkeys had 24-hour access to water and were fed manufactured chow and various fruits and vegetables daily between 1600 and 1800 hours. Additionally, monkeys were routinely tested on computer testing systems, from which they could earn food pellets. This study complied with protocols approved by the Georgia State University IACUC.
Materials
The monkeys were tested using the Language Research Center’s Computerized Test System comprising a personal computer, digital joystick, color monitor, and pellet dispenser (see Evans, Beran, Chan, Klein and Menzel 2008). Monkeys manipulated the joystick to produce isomorphic movements of a computer-graphic cursor on the screen. Contacting appropriate computer-generated stimuli with the cursor brought them a 45-mg banana-flavored chow pellet (Bio-Serv, Frenchtown, NJ) using a pellet dispenser interfaced to the computer through a digital I/O board (PDISO8A; Keithley Instruments, Cleveland, OH). All monkeys had previously participated in multiple psychological experiments involving this computerized test system (e.g., Beran 2008; Beran, Decker, Schwartz and Schultz 2011; Beran et al. 2008a, 2008b; Beran and Smith 2011).
Design and Procedure
The monkeys first learned to select the stimuli used in this experiment in a pre-determined order. Half of the monkeys were given a sequence of red letters - A, B, C, D, and E, and the other half of the monkeys were given a sequence of black symbols - #, $, @, &, and %. The use of two unique sets of stimuli was designed as part of an experimental study not related to the present study. Monkeys had no prior experience with either sets of selected stimuli. Stimuli were always presented in randomized locations around the perimeter of the screen, and the cursor was presented in the center of the screen. The monkeys contacted stimuli using a joystick-controlled cursor. If the animals contacted the correct stimulus, it disappeared from the screen, and the cursor returned to the center of the screen if more stimuli remained to be selected. When the stimulus selected was not the correct selection, the computer produced a buzz tone, and a 20 second timeout occurred during which the screen was blank. Then, the next trial was presented. After a monkey selected all stimuli in the correct order for a trial, the computer produced a melodic tone, and the monkey received a pellet dispensed by a mechanized food dispenser connected to the computer and controlled by the program.
Training
Initially, only two stimuli were presented. When a monkey was correct on 16 of the most recent 20 trials, the number of stimuli increased to three, and so on throughout these training sessions. Monkeys continued in the training phase until they reached a stable pattern of responding across three or more sessions in which they met the criterion outlined above with the same number of stimuli, up to a maximum of five stimuli. In other words, we trained them until there was stability across sessions regarding how many stimuli they could reliably sequence. Each monkey had a minimum of seven training sessions and a maximum of 10 (in the cases in which some sessions for an animal consisted of low trial counts, additional sessions were given). Liam, Logan, Nala, Wren, and Gambit were proficient with five stimuli, and Drella, Griffin, and Lily were proficient with four stimuli. Respectively, these were the numbers of stimuli used with each of these monkeys throughout the testing phase.
Testing
The testing phase consisted of a mix of baseline and shift trials. Baseline trials were exactly like those outlined above, where all stimuli appeared at trial outset, and remained visible and in the same locations throughout the trial, although the locations of the stimuli varied across trials. During shift trials, as the cursor contacted the correct first stimulus, the positions of the second and third stimuli were interchanged on the screen. This interchange occurred through the disappearance of those stimuli and their rapid reappearance in each other’s position. The shift occurred very quickly with no perceptible time delay. These trials otherwise were identical to the non-shift trials with the requirements for correct completion of the trial remaining the same – selecting all stimuli in the correct order.
Shift trials occurred on every tenth trial within a session, with the remaining nine trials coming from the baseline condition. The monkeys completed 1,000 trials each - 900 trials were baseline trials, and 100 trials involved shift occurring for the second and third stimuli in the sequence. Because of a program error, one monkey, Lily, completed only 620 trials, but her data were still partially analyzed.
Results
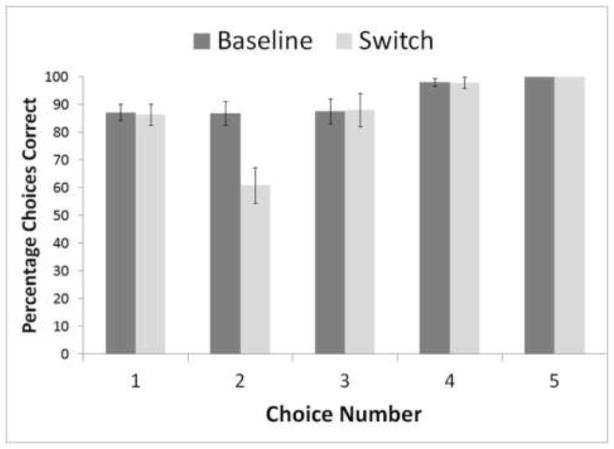
Two analyses were relevant to the question of planning by these monkeys when comparing performance in baseline to performance in which the second and third stimuli to be selected were shifted in location. First, we predicted that performance would be significantly lower after a shift than in baseline, and this was confirmed (Figure 1). There were no differences in performance for first, third, or fourth selections (fifth selections and fourth selections for some monkeys were always at 100% because only one stimulus remained for selection). However, second selections led to far more errors when a shift occurred than when it did not. This was true for every individual except one: Griffin χ2 (1, N = 865) = 35.10, p < 0.001; Lily χ2 (1, N = 526) = 17.18, p < 0.001; Gambit χ2 (1, N = 913) = 45.65, p < 0.001; Liam χ2 (1, N = 886) = 177.32, p < 0.001; Logan χ2 (1, N = 924) = 43.48, p < 0.001; Nala χ2 (1, N = 904) = 102.23, p < 0.001; Wren χ2 (1, N = 836) = 276.65, p < 0.001. One monkey, Drella, did not show the anticipated effect, performing at 73% correct in baseline and 68% correct on shift trials for his second selections; Drella χ2(1, N = 795) < 1.0, ns.
Figure 1.
The percentages of correct choices at each serial position for baseline and shift trials. Error bars are 95% confidence intervals.
We analyzed all trials in which the second selection was made in error, with the prediction that these responses should have been made to the third item in the sequence (which was transposed to where the correct, second item had been) at levels significantly higher than would be expected by chance selections among the erroneous remaining stimuli. Thus, for Drella and Griffin chance was 50% (the third and fourth items were the only possible errors that could be made if the second item was not selected). For the remaining monkeys, chance was 33% because there were three possible errors (the third, fourth, or fifth item could be selected). [This analysis was not possible with Lily because of the program error.] Every monkey selected the third item in the sequence more often than should have occurred by chance – Drella: 75.0%; Griffin: 90.3%; Gambit: 84.85%; Liam: 90.38%; Logan: 96%; Nala: 100%; Wren: 93.94%, all p < 0.05, binomial test.
We also analyzed the response times of the animals for correct selection of the second stimulus as a function of trial type (baseline or shift). If monkeys were noticing that the location they had planned to select next no longer held the correct stimulus, it should take additional time to look for and move to that stimulus. However, if monkeys were not planning but were looking for each correct choice at each step in the trials, there should be no difference in the response time for the selection of the second item for baseline trials and for shift trials. These mean response times for each condition for the second selection are presented in Figure 2. Any trials with response durations exceeding 10 seconds were eliminated from this analysis, because these are considered outlier response times and likely reflect that the monkey disengaged from the task for other reasons (e.g., distraction, water consumption). For six of the eight monkeys, there was a significant effect of trial type on response time, Griffin F (1, 736) = 44.27, p < 0.001; Gambit F (1, 911) = 8.75, p = 0.003; Liam F (1, 884) = 7.42, p = 0.007; Logan F (1, 851) = 4.14, p = 0.042; Nala F (1, 802) = 39.48, p < 0.001; Wren F (1, 674) = 21.78, p < 0.001. Two monkeys did not show this effect, Drella F (1, 793) = 0.85, p = 0.35; Lily F (1, 520) = 0.07, p = 0.79.
Figure 2.
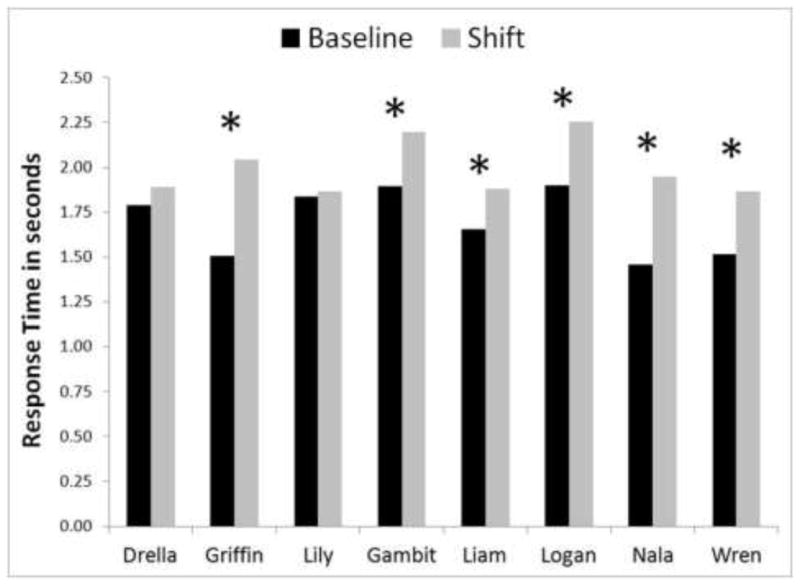
The response times for selecting correctly the second stimulus for shift and baseline trials. Asterisks indicate a significant difference in response times for a monkey between those two trial types.
Discussion
Capuchin monkeys looked very similar to previously tested chimpanzees and rhesus monkeys (Beran et al. 2004) in their response patterns on shift trials. After learning to select stimuli in sequence, most of the monkeys showed many more errors in their second selections after the second and third stimuli in the sequence were transposed in location. Beyond that, even for correct responses, for most monkeys, the time required to make a correct second selection was significantly longer after a transposition than in trials without one. These patterns suggest that the monkeys were planning sequences of responses. However, this test is not sufficient to demonstrate how extensive the planned sequence was for the monkeys. To determine this, mask trials were needed so that it would be clear how far into a planned sequence the monkeys would go at the outset of trials. Again, previous research has been unclear on this issue, with some studies showing performance that would seem to indicate that the entire sequence was planned (e.g., Kawai and Matsuzawa 2000), whereas other studies show much more limited planning, and mainly just for one selection beyond the current one (e.g., Beran et al. 2004; Scarf et al. 2011). Experiment 2 tested this in the capuchin monkeys.
Experiment 2
Experiment 2 introduced the masking procedure in an attempt to determine if capuchin monkeys were processing all stimuli in the array at trial outset and perhaps implementing a planned response sequence. If this was true, they should perform at above chance levels in selecting all masked stimuli as has been reported for a chimpanzee (Kawai and Matsuzawa 2000). Alternatively, they may have shown no evidence of planning, or limited evidence such as that reported for some chimpanzees, rhesus monkeys, and pigeons (Beran et al. 2004; Scarf and Colombo 2009, 2010).
Method
Participants
All monkeys in Experiment 1 also participated in Experiment 2.
Materials
The apparatus was the same as in Experiment 1.
Design and Procedure
The testing phase consisted of a mix of baseline and mask trials. Baseline trials were exactly like those outlined in Experiment 1. For each block of three consecutive trials, the first two trials were baseline trials in which all stimuli remained on the screen, uncovered, throughout the trial. The third trial in each block was a mask trial. During mask trials, when the monkeys contacted the first stimulus correctly, all remaining stimuli on the screen were masked with opaque rectangular boxes of equal size so that the stimuli were no longer visible. All monkeys except for one performed 3,000 trials each (approximately 2,000 baseline trials and 1,000 mask trials). One monkey, Gambit, was accidentally stopped in the experiment after completing only 1,162 trials, but her performance was analyzed similarly to the other monkeys.
Results
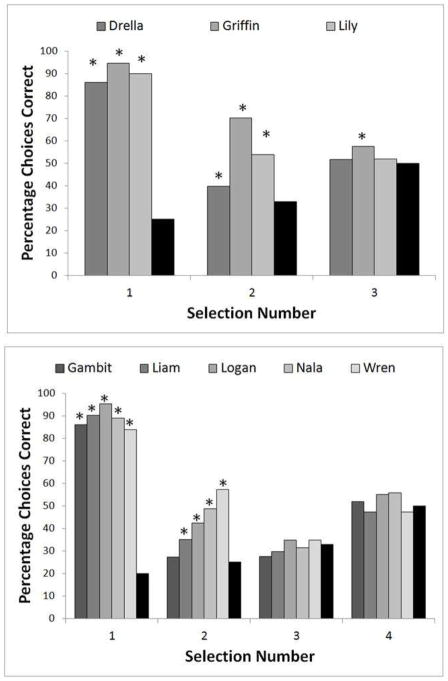
All monkeys performed at levels significantly better than chance for every selection during baseline trials (all p < 0.001) – Selection 1 range: 82.87–98.15% correct; Selection 2 range: 87.4–97.1% correct; Selection 3 range: 92.2–99.0% correct; Selection 4: 97.7–99.5% correct. The performance of each animal for each position with mask trials is presented in Figure 3. As would be expected, all monkeys performed at levels significantly better than chance for the first selection during mask trials as it was never covered by an opaque box (all p < 0.001, binomial test). Additionally, all monkeys except for one selected the second stimulus at levels significantly better than chance during mask trials (p < 0.01). Gambit did not exceed chance levels on the second selection. With only one exception (Griffin’s selection of the third stimulus), none of the monkeys selected any of the additional stimuli at levels significantly better than chance.
Figure 3.
The percentage correct responses at each selection position for mask trials. Black bars show the expected chance level of performance. Graphs are divided by animals that were presented with four stimuli or five, and asterisks indicate performance levels that exceeded chance.
To determine whether performance improved over time, we also looked at only the last 100 mask trials for each monkey. The performance pattern matched that shown in Figure 3 exactly where each monkey was significantly better than chance for the same selections, but not for any additional selections. Thus, performance in the last 100 trials was not different from the overall performance of the monkeys indicating that the monkeys did not improve over time.
Discussion
The data from this experiment indicate that all of the monkeys were planning selections, but as was previously reported for chimpanzees and rhesus monkeys (Beran et al. 2004), planning was restricted to one selection only. Thus, capuchin monkeys look very similar to Old World monkeys and some apes in their performance on this version of the computerized planning task. Despite being well-trained on the sequence, and despite getting mask trials frequently, the monkeys did not generate strategies that would allow them to remember and select the stimuli in the correct order after the masks were applied, and this led to many errors.
General Discussion
Results from the current study indicate that this New World capuchin monkey species (Cebus apella) sequenced visual stimuli in a learned order, planning their subsequent selections to a certain degree. These capuchin monkeys planned only their next, immediate response when selecting stimuli rather than planning the entire sequence of however many stimuli they were presented. These results are highly comparable to performance by Old World primate species who also demonstrate similar limited proficiencies in planning responses of serially ordered stimuli (Beran et al. 2004). Capuchin monkeys performed more similarly to chimpanzees in this task than in some previous experiments that used other tests to assess planning (e.g., Fragaszy et al. 2009). In fact, these data now provide a more robust survey of this form of planning capacity across New World, Old World, and great ape species. And, given the very similar results with pigeons (e.g., Scarf and Colombo 2010), the paradigm can be considered even more broad and yet still consistent in its outcome.
The monkeys were given two kinds of tests to assess the extent to which they would plan sequences of responses. Shift trials occurred in which the second and third stimuli were transposed to test whether the monkeys planned their next response prior to making their selection. The majority of capuchin monkeys (six of eight) performed markedly worse when the locations of stimuli were interchanged in comparison to baseline trials, and took significantly longer to complete these trials, even in correct shift trials. The increase in both error rate and response times for shift trials is indicative of planning of sequentially presented items. Additionally, errors in the shift trials were primarily made to the third item in the sequence confirming that the monkeys were anticipating their next move to a location where the item them was transposed. Mask trials occurred when all remaining stimuli were covered by opaque markers after the first stimulus was selected, and seven of eight capuchin monkeys exceeded chance for the first and second selections on a trial. This indicates that capuchin monkeys were limited in their ability to plan ahead for sequentially presented items, encoding the location of only the next stimulus, rather than the entire set.
Performance in sequential responding and planning tasks is typically explained by one of two accounts. The serial search strategy involves selecting each response separately based on the remaining available stimuli in the environment. In contrast to this, the collective search strategy involves the simultaneous processing of all given stimuli available, which is then encoded prior to the beginning of any motor responses (Oshiba, 1997). The capuchin monkeys did not appear to be using either of these two strategies outlined. Instead, like chimpanzees and rhesus monkeys (see Beran et al. 2004), the capuchin monkeys may have been using a very limited form of planning that only involved searching for one subsequent selection while simultaneously making a current selection. For example, while moving to stimulus A, they must have discerned where B was located because they chose that stimulus at above chance levels even when it was masked. But, their strategy would not work any further, because as they were moving to B, masks were on all remaining stimuli, and so chance performance occurred for subsequent responses.
There is a second alternative. Rather than planning the full sequence, the monkeys may have instead encoded the first two responses in the serial order at the trial outset by discerning both of those locations. This would be a process that involved (slightly) more planning because multiple responses would be anticipated before any response was started. Our data cannot distinguish between those alternatives, but other data can. Scarf and Colombo (2009) used eye-tracking software with macaque monkeys and found no evidence that the monkeys were searching out multiple response locations before beginning their response pattern. Instead, they also reported that monkeys often were looking at the second stimulus in the sequence while responding to the first. They also reported that the latency to respond to an item in their sequencing task was the same whether a monkey had seen that item or not during its initial scan of the array. All of these patterns suggest that planning is very limited in this kind of task and lend support to the first alternative of searching for one subsequent selection while simultaneously making a current selection.
Although the monkeys did not plan the full sequence, we were more interested in how a New World primate species, particularly the large-brained capuchin monkey, performed in a sequential responding and planning task, and how their performance compared to previous data from Old World primate species. Planning of behaviors, even for relatively brief time intervals such as planning of sequential motor responses, is beneficial as it facilitates speed and accuracy in subsequent decision-making. For the current task, monkeys certainly could optimize their reward intake by attempting to locate the next response while moving to the currently discerned next stimulus in the sequence. This would save time, and still allow for correctly ordering stimuli, at least when they remained visible throughout and were not spatially transposed.
Capuchin monkeys’ equivalent performance in the current task is perhaps not surprising as this is not the only cognitive capacity held in common with Old World primate species. Capuchin monkeys often perform similarly as great apes and rhesus macaques in tests of physical cognition (Beran 2008; Beran et al. 2008a, 2008b; D’Amato and Colombo 1988; Evans, Beran and Addessi 2010; Evans and Westergaard 2004; Flemming 2011; Judge, Evans and Vyas 2005; Kennedy and Fragaszy 2008; McGonigle, Chalmers and Dickinson 2003; Poti et al. 2010; Wright and Katz 2006; Yocum and Boysen 2010). However, this New World monkey species has also demonstrated significant differences from other species in higher-order cognitive processes such as memory monitoring and metacognition (e.g., Beran et al. 2009; Fujita 2009). This makes the current comparison a relevant topic of inquiry given the assumption that planning abilities and metacognitive abilities might be related given their joint inclusion under the umbrella term “executive functions” or “higher-order cognitive skills”. The present results would seem to indicate that differences in metacognitive-like performances in macaques and chimpanzees versus capuchin monkeys are likely not related to a form of monitoring that is required to anticipate a response and then implement that response. For example, capuchin monkeys are likely not failing at information-seeking tasks because they are unable to plan to access additional information, but perhaps because they do not know that they even need additional information. Rather, differences in metacognitive task performance across nonhuman primate species may yet reflect differences in the ability to gain access to one’s own mental states and then act on that accessed information, although a number of alternate hypotheses remain to be tested. For example, differences in behavioral inhibition might exist among primate species, and such inhibition might be necessary to allow for uncertainty responses to be made or for information seeking responses to be generated in place of primary responses.
More data are needed to determine the extent to which capuchin monkeys perform similarly on other planning tasks that are more often (or even exclusively) used with apes or Old World monkeys. Further investigations into more complex forms of planning in the capuchin monkey would be both informative and worthwhile given the current results and will be critical to understanding the breadth of these capabilities across the order Primates. Given the close performance patterns across primate species in the planning task used in this study, capuchin monkeys should be viewed as a useful species for other tests of planning, prospection, and other executive functions, even though they may not have access to the full suite of executive functions that are available to those other species.
Acknowledgments
Michael J. Beran and Audrey E. Parrish, Language Research Center and Department of Psychology, Georgia State University.
This research was supported by National Science Foundation grant BCS - 0924811 and National Institutes of Health Grant HD – 060563 and a Georgia State University Brains and Behavior Graduate Fellowship to AEP. We thank Ted Evans and Bonnie Perdue for their assistance in conducting the experimental sessions with the monkeys, and for reading earlier versions of this paper.
References
- Adams A, Santi A. Pigeons exhibit higher accuracy for chosen memory tests than for forced memory tests in duration matching-to-sample. Learn Behav. 2011;39:1–11. doi: 10.1007/s13420-010-0001-7. [DOI] [PubMed] [Google Scholar]
- Ardila A. On the evolutionary origins of executive functions. Brain and Cognition. 2008;68:92–99. doi: 10.1016/j.bandc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Anim Cogn. 2009;12:169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ. Capuchin monkeys (Cebus apella) succeed in a test of quantity conservation. Anim Cogn. 2008;11:109–116. doi: 10.1007/s10071-007-0094-3. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Decker S, Schwartz A, Schultz NB. Monkeys (Macaca mulatta and Cebus apella) and human adults and children (Homo sapiens) enumerate and compare subsets of moving stimuli based on numerosity. Frontiers Comp Psychol. 2011;2:Article 61. doi: 10.3389/fpsyg.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Harris EH, Evans TA, Klein ED, Chan B, Flemming TJ, Washburn DA. Ordinal judgments of symbolic stimuli by capuchin monkeys (Cebus apella) and rhesus monkeys (Macaca mulatta): The effects of differential and nondifferential reward. J Comp Psychol. 2008;122:52–61. doi: 10.1037/0735-7036.122.1.52. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Klein ED, Evans TA, Chan B, Flemming TJ, Harris EH, Washburn DA, Rumbaugh DM. Discrimination reversal learning in capuchin monkeys (Cebus apella) Psych Record. 2008;58:3–14. [Google Scholar]
- Beran MJ, Pate JL, Washburn DA, Rumbaugh DM. Sequential responding and planning in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta) J Exp Psychol Anim Behav Proc. 2004;30:203–212. doi: 10.1037/0097-7403.30.3.203. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Smith JD. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Cognition. 2011;120:90–105. doi: 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Coutinho MVC, Couchman JJ, Boomer J. The psychological organization of “uncertainty” responses and “middle” responses: A dissociation in capuchin monkeys (Cebus apella) J Exp Psychol Anim Behav Proc. 2009;35:371–381. doi: 10.1037/a0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro D, Matsuzawa T. Numerical ordering in a chimpanzee (Pan troglodytes): Planning, executing, and monitoring. J Comp Psychol. 1999;113:178–185. [Google Scholar]
- Call J. Do apes know that they could be wrong? Anim Cogn. 2010;13:689–700. doi: 10.1007/s10071-010-0317-x. [DOI] [PubMed] [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Anim Cogn. 2001;4:207–220. [Google Scholar]
- D’Amato MR, Colombo M. Representation of serial order in monkeys (Cebus apella) J Exp Psychol Anim Behav Proc. 1988;14:131–139. [PubMed] [Google Scholar]
- D’Amato MR, Colombo M. Serial learning with wild card items by monkeys (Cebus apella): Implications for knowledge of ordinal position. J Comp Psychol. 1989;103:252–161. doi: 10.1037/0735-7036.103.3.252. [DOI] [PubMed] [Google Scholar]
- Darwin C. The decent of man and selection in relation to sex. Appleton and Company; New York: 1871. [Google Scholar]
- Evans TA, Beran MF, Addessi E. Can nonhuman primates use tokens to represent and sum quantities? J Comp Psychol. 2010;124:369–380. doi: 10.1037/a0019855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behav Res Methods. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Evans TA, Westergaard GC. Self-control and tool use in tufted capuchin monkeys (Cebus apella) J Comp Psychol. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. [DOI] [PubMed] [Google Scholar]
- Flemming TM. Conceptual thresholds for same and different in old(Macaca mulatta) and new-world (Cebus apella) monkeys. Behav Proc. 2011;86:316–322. doi: 10.1016/j.beproc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AL, Crystal JD. Metacognition in the rat. Curr Biol. 2007;17:551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AL, Crystal JD. “Play it again”: a new method for testing metacognition in animals. Anim Cogn. 2012;15:187–199. doi: 10.1007/s10071-011-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragaszy D, Johnson-Pynn J, Hirsh E, Brakke K. Strategic navigation of two-dimensional alley mazes: Comparing capuchin monkeys and chimpanzees. Anim Cogn. 2003;6:149–160. doi: 10.1007/s10071-002-0137-8. [DOI] [PubMed] [Google Scholar]
- Fragaszy D, Kennedy EH, Murnane A, Menzel CR, Brewer G, Johnson-Pynn J, Hopkins WD. Navigating two-dimensional mazes: Chimpanzees (Pan troglodytes) and capuchins (Cebus apella sp.) profit from experience differently. Anim Cogn. 2009;12:491–504. doi: 10.1007/s10071-008-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K. Metamemory in tufted capuchin monkeys (Cebus apella) Anim Cogn. 2009;12:575–585. doi: 10.1007/s10071-009-0217-0. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proc Nat Acad Sci. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: A test with pigeons. J Exp Psychol Anim Behav Proc. 1999;25:389–395. [Google Scholar]
- Inoue S, Matsuzawa T. Working memory of numerals in chimpanzees. Curr Biol. 2007;17:R1004–R1005. doi: 10.1016/j.cub.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Judge PG, Evans TA, Vyas DK. Ordinal representation of numeric quantities by brown capuchin monkeys (Cebus apella) J Exp Psychol Anim Behav Proc. 2005;31:79–94. doi: 10.1037/0097-7403.31.1.79. [DOI] [PubMed] [Google Scholar]
- Kawai N, Matsuzawa T. Numerical memory span in a chimpanzee. Nature. 2000;403:39–40. doi: 10.1038/47405. [DOI] [PubMed] [Google Scholar]
- Kennedy EH, Fragaszy DH. Analogical reasoning in a capuchin monkey (Cebus apella) J Comp Psychol. 2008;122:167–175. doi: 10.1037/0735-7036.122.2.167. [DOI] [PubMed] [Google Scholar]
- Kornell N, Son LK, Terrace HS. Transfer of metacognitive skills and hint seeking in monkeys. Psych Sci. 2007;18:64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- McGonigle B, Chalmers M, Dickinson A. Concurrent disjoint and reciprocal classification by Cebus apella in seriation tasks: Evidence for hierarchical organization. Anim Cogn. 2003;6:185–197. doi: 10.1007/s10071-003-0174-y. [DOI] [PubMed] [Google Scholar]
- Miller GA, Galanter E, Pribram KH. Plans and the structure of behavior. Adams, Bannister, Cox; New York: 1986. [Google Scholar]
- Mushiake H, Saito N, Sakamoto K, Sato Y, Tanji J. Visually based path-planning by Japanese monkeys. Cog Brain Res. 2001;11:165–169. doi: 10.1016/s0926-6410(00)00067-7. [DOI] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Why investigate metacognition? In: Metcalfe J, Shimamura AP, editors. Metacognition. Knowing about knowing. MIT Press; Cambridge, MA: 1994. pp. 1–25. [Google Scholar]
- Oshiba N. Memorization of serial items by Japanese monkeys, a chimpanzee, and humans. Japanese Psychological Research. 1997;39:236–252. [Google Scholar]
- Paukner A, Anderson JR, Fujita K. Redundant food searches by capuchin monkeys (Cebus apella): A failure of metacognition? Anim Cogn. 2006;9:110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Poti P, Kanngiesser P, Saporiti M, Amiconi ABB, Call J. Searching in the middle—capuchins’ (Cebus apella) and bonobos’ (Pan paniscus) behavior during a spatial search task. J Exp Psychol Anim Behav Proc. 2010;36:92–109. doi: 10.1037/a0015970. [DOI] [PubMed] [Google Scholar]
- Scarf D, Colombo M. Eye movements during list execution reveal no planning in monkeys (Macaca fascicularis) J Exp Psychol Anim Behav Proc. 2009;35:587–592. doi: 10.1037/a0014020. [DOI] [PubMed] [Google Scholar]
- Scarf D, Colombo M. The formation and execution of sequential plans in pigeons (Columba livia) Behav Proc. 2010;83:179–182. doi: 10.1016/j.beproc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Scarf D, Danly E, Morgan G, Colombo M, Terrace HS. Sequential planning in rhesus monkeys (Macaca mulatta) Anim Cogn. 2011;14:317–324. doi: 10.1007/s10071-010-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Redford JS, Washburn DA. Dissociating uncertainty responses and reinforcement signals in the comparative study of uncertainty monitoring. J Exp Psychol Gen. 2006;135:282–297. doi: 10.1037/0096-3445.135.2.282. [DOI] [PubMed] [Google Scholar]
- Suda-King C. Do orangutans (Pongo pygmaeus) know when they do not remember? Anim Cogn. 2008;11:21–42. doi: 10.1007/s10071-007-0082-7. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Shettleworth SJ. Memory without awareness: Pigeons do not show metamemory in delayed matching to sample. J Exp Psychol Anim Behav Proc. 2008;34:266–282. doi: 10.1037/0097-7403.34.2.266. [DOI] [PubMed] [Google Scholar]
- Terrace HS. A nonverbal organism’s knowledge of ordinal position in a serial learning task. J Exp Psychol Anim Behav Proc. 1986;12:203–214. [Google Scholar]
- Washburn DA. Analyzing the path of responding in maze-solving and other tasks. Behav Res Meth Inst Comp. 1992;24:248–252. doi: 10.3758/bf03203502. [DOI] [PubMed] [Google Scholar]
- Wright AA, Katz JS. Mechanisms of same/different concept learning in primates and avians. Behav Proc. 2006;72:234–254. doi: 10.1016/j.beproc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Yocom AM, Boysen ST. Capuchins (Cebus apella) can solve a means-ends problem. J Comp Psychol. 2010;124:271–277. doi: 10.1037/a0019369. [DOI] [PubMed] [Google Scholar]