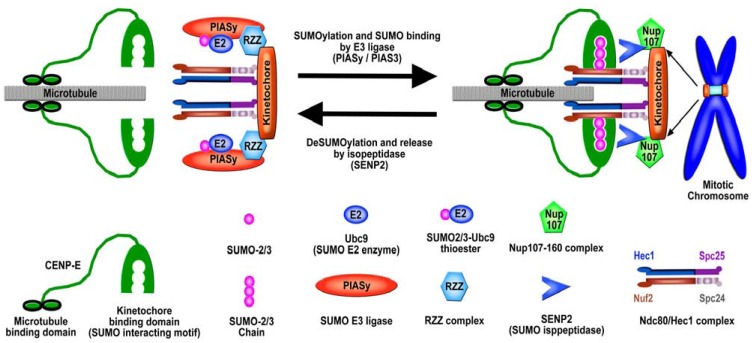
Fig. (3).
A model shows that polymeric SUMO-2/3 chain modification of kinetochore proteins regulates CENP-E localization to the kinetochores. The kinetochore-associated microtubule motor protein CENP-E contains the NH2-terminal microtubule binding domain, the rod domain, and the COOH-terminal kinetochore binding domain. CENP-E forms a dimer through its rod domain and is targeted to the kinetochore through its kinetochore binding domain. The kinetochore binding domain of CENP-E also contains a polymeric SUMO-2/3 chain interacting motif (notches) that is essential for CENP-E localization to the kinetochores. Furthermore, the known CENP-E-interacting proteins, Nuf2 and BubR1, have been shown to be specifically modified by SUMO-2/3 (purple circles). The Nuf2 is one of the subunits of the Ndc80/Hec1 complex, including Hec1, Nuf2, Spec24 and Spec25. Moreover, both SUMO E3 ligases, PIASy and PIAS3, are localized to unattached kinetochores during early mitosis. PIASy is targeted to the unattached kinetochores by the Rod/Zw10/Zwilch (RZZ) complex, recruits the SUMO thioester-loaded Ubc9 (SUMO E2 conjugating enzyme), and stimulates the poly-SUMO-2/3 chain modification of kinetochore proteins, such as Nuf2 and BubR1 (not shown here), leading to the kinetochore localization of CENP-E and the microtubule attachment to kinetochores. On the other hand, the SUMO isopeptidase SENP2 is hypothesized to be recruited to kinetochores through its interaction with the Nup107-160 complex, leading to the deSUMOylation of Nuf2 and BubR1 and the dissociation of CENP-E from kinetochores. Therefore, SUMOylation and deSUMOylation of the kinetochore proteins regulate the association and dissociation of CENP-E with kinetochores and thereby the microtubule attachment to the chromosome.