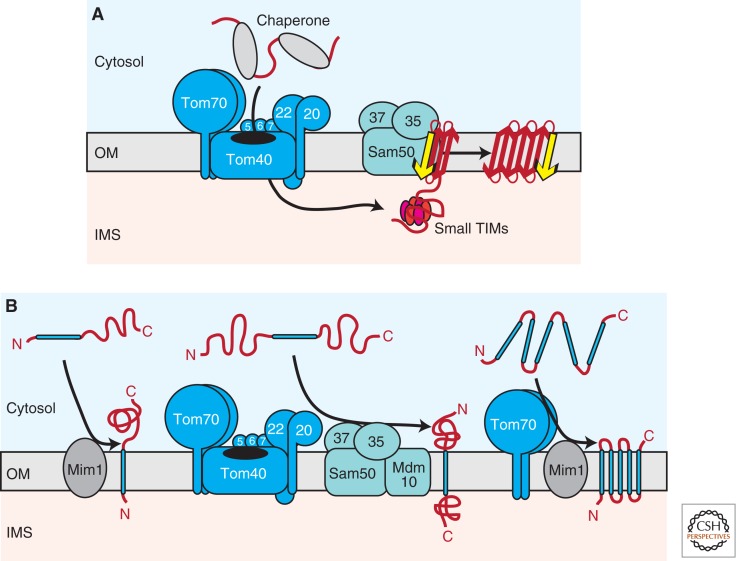
Figure 2.
Biogenesis of outer membrane proteins. (A) Several mitochondrial outer membrane proteins are characterized by a β-barrel topology. On passage of the TOM complex, β-barrel precursors are guided through the aqueous IMS by hexameric small TIM complexes to the SAM complex. A specific import signal (β-signal), which is located within the last β-strand of the precursor, is recognized by the Sam35 receptor. Sam50 forms a central cavity in the SAM complex, where membrane insertion and folding of precursors takes place. (B) Multiple pathways mediate the insertion of α-helical proteins into the outer membrane. Signal-anchored precursors require Mim1 for membrane integration (left). The biogenesis of Tom22, which contains a single central transmembrane segment, depends on both the TOM and SAM–Mdm10 complexes (middle). Polytopic α-helical outer membrane proteins are recognized by the Tom70 receptor and transferred to Mim1 for membrane insertion (right). OM, outer membrane; IMS, intermembrane space; red arrow, β-strand; yellow arrow, β-signal; blue bar, transmembrane α-helix C, carboxy; N, amino.