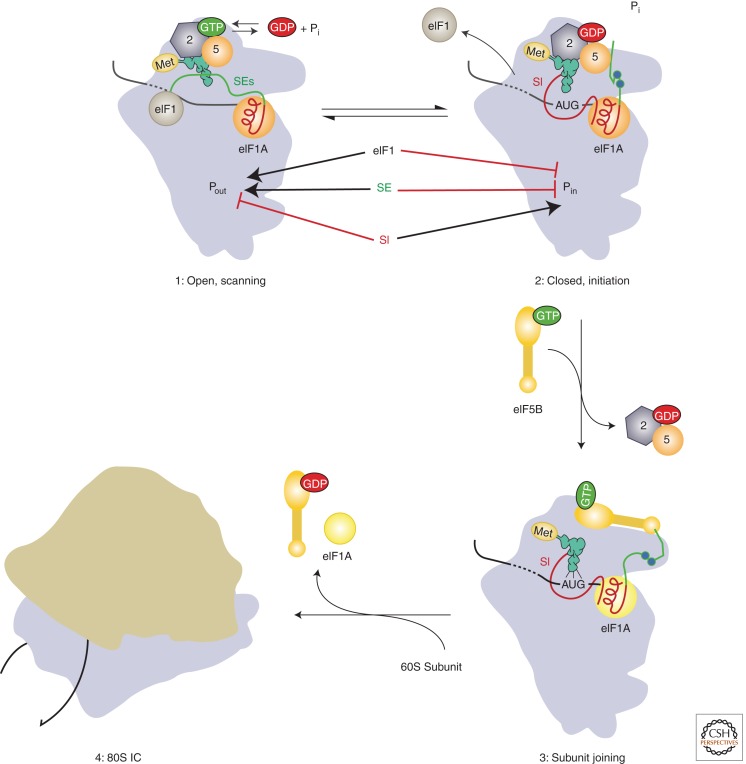
Figure 3.
Model of the roles of eIF1A’s amino- and carboxy-terminal tails in mediating start codon recognition and later steps in eukaryotic translation initiation. Before start codon recognition (complex 1) the amino- and carboxy-terminal tails (CTTs; shown in red and green, respectively) are both in the P site of the 40S subunit. On start codon recognition (complex 2), the initiator tRNA moves from the Pout to Pin state, causing both eIF1 and the CTT of eIF1A to be ejected from the P site. eIF1 stabilizes the open conformation of the PIC and the Pout state of the initiator tRNA. The scanning enhancer (SE) elements in the CTT of eIF1A (shown as blue balls) stabilize the open state of the PIC relative to the closed state. Conversely, the scanning inhibitor (SI) element in eIF1A’s NTT destabilizes the open state, thus promoting closed complex formation. The CTT of eIF1A may interact directly with eIF5 after start codon recognition, and it is hypothesized that this interaction triggers Pi release from eIF2. After Pi release and dissociation of eIF2·GDP and eIF5 from the PIC (complex 3), the CTT of eIF1A is free to interact with eIF5B·GTP, recruiting it to the complex and promoting subunit joining. Release of eIF5B·GDP and eIF1A from the resulting 80S IC produces the final translation-competent ribosome, poised at the start codon to commence decoding of the mRNA. (Modified from Hinnebusch 2011; reproduced, with permission.)