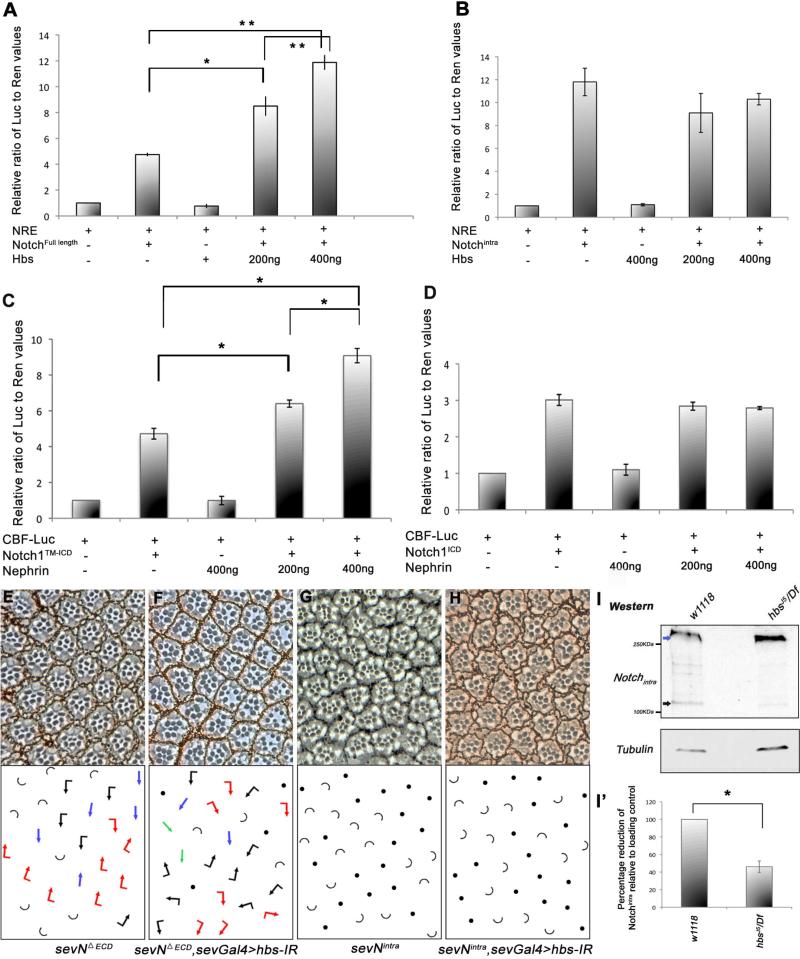
Figure 5. hbs is required for the activity of membrane-tethered Notch.
(A-B) Activation of N-signaling induced luciferase reporter containing Su(H) binding sites (NREs) in S2 cells co-transfected with Hbs along with either full length N (A) or NICD (B). Hbs expression causes an increase in activity of full length N in a dose dependent manner, while reporter activity induced by intracellular NICD remains unaffected by Hbs. Values represent mean ratio of luciferase/renilla control (internal control). Representative experiment from three independent experiments is shown (error bars represent standard deviations within each experiment). P-values were *P<0.05 and **P<0.005 (student t-test).
(C-D) Activation of mammalian CBF Notch luciferase reporter in 293T cells co-transfected with Nephrin along with either mN1TM-ICD (C) or mN1ICD (D); co-expression of Nephrin enhanced mN1TM-ICD based CBF reporter activation in a dose dependent manner (*P<0.005), while mN1ICD mediated reporter induction remained unchanged. Error bars denote standard deviations within each experiment.
(E-H) Tangential sections of adult eyes of indicated genotypes, anterior is left and dorsal up. Arrows are as in Figure 1, with blue arrows representing R4-R4 type symmetrical clusters and half circles ommatidia with >1 R7 (at the expense of R1/R6, as frequently observed in N overactivation caused by transformation of R1 and/or R6 to R7; Fortini et al., 1993; Tomlinson and Struhl, 1999).
(E-F) Knock-down of hbs (sevGal4>hbs-IR) markedly suppresses the phenotype of membrane tethered sevNΔECD (compare E and F; note even some R3-R3-type clusters; quantified in Suppl. Figure S4),
(G-H) The effect of “active” cytoplasmic sev-Nintra (G) is not affected by hbs-IR (H), suggesting a requirement of Hbs at the membrane.
(I-I’) Western blot of third instar larval eye-brain complexes showing cleavage pattern of endogenous N protein. In wild-type larvae higher molecular weight full-length N (blue arrow) and smaller cleavage products, N-intra fragments (~120K, black arrow) are detected. In samples from hbs mutants, there is a significant reduction in the levels of N cleavage fragments, whereas levels of full-length N protein are unchanged (γ-Tubulin, lower panel, serves as loading control). (I’) Quantification showing percentage reduction of Notchintra cleavage products (black arrows), which are reduced in hbs mutant larval eye-brain complexes (error bars represent standard deviations with*p<0.01).
See also Suppl. Figure S4.