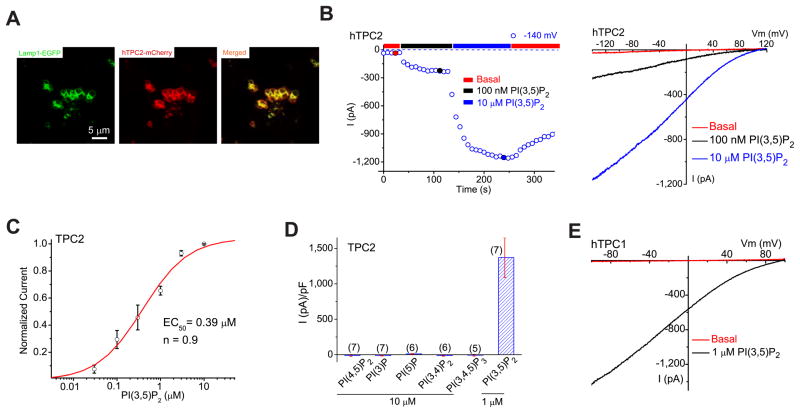
Figure 2. PI(3,5)P2 activates recombinant TPCs in endolysosomes.
(A). TPC2 proteins are localized in Lamp1-positive late endosomes and lysosomes in COS-1 cells that were transfected with TPC2 and Lamp-1 fusion proteins and treated with vacuolin-1. (B). PI(3,5)P2 activated a large whole-endolysosome current with Erev > + 80mV in an EGFP (hTPC2)-positive endolysosome isolated from an hTPC2-EGFP-transfected COS-1 cell. Whole-endolysosome currents were elicited by repeated voltage ramps (−140 to +140 mV; 400 ms) with a 4s interval between ramps; current amplitudes measured at −140 mV were used to plot the time course of activation. The right panel shows representative I-V traces of hTPC2-mediated whole-endolysosome currents (IhTPC2) before (red; −20 ± 4pA/pF at −120 mV, n = 9) and after (black and blue) PI(3,5)P2 bath application at three different time points, as indicated in the left panel (red, blue and black circles). Only a portion of the voltage protocol is shown; holding potential (HP) = 0 mV. (C). Dose-dependence of PI(3,5)P2-dependent activation (EC50 = 390 ± 94 nM, Hill slope (n) = 0.9, n= 13 vacuoles). (D). Specific activation of TPC2 by PI(3,5)P2 (in 1 μM), but not other PIPs (all in 10 μM). On average, ITPC2 in the presence of 1μM PI(3,5)P2 was 1410 ± 360pA/pF at −120mV (n = 7). (E). Activation of IhTPC1 by 1 μM PI(3,5)P2. Data are presented as the mean ± SEM. See also Figure S2.