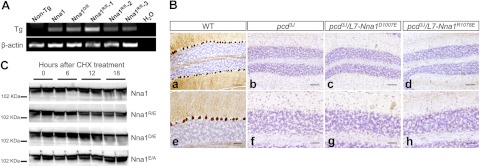
Figure 4.
Two amino acids uniquely conserved between the Nna1 family and ASPA are essential for Nna1 activity. A) Representative RT-PCR of total RNA from cerebellum from wild-type (non-Tg, nontransgenic) and transgenic mice harboring mutations of D1007 (Nna1D/E) and R1078E (Nna1R/E) as well as transgenic mice expressing wild-type Nna1 (Nna1) at a level that rescues Purkinje cell degeneration. Identical mutations in ASPA homologous to D1007 and R1078 cause Canavan disease. B) Calbindin D-28K immunohistochemistry and hematoxylin counterstaining of cerebellum from 2-mo-old wild-type mice (a, e), pcd3J mice (b, f), and pcd3J/L7-Nna1D1007E (c, g) and pcd3J/L7-Nna1R1078E (d, h) transgenic mice reveals that neither mutant rescues Purkinje cell degeneration, which suggests that D1007 and R1078 are essential for activity of Nna1 in vivo and that Nna1 and ASPA have similar catalytic properties. Scale bars = 100 μm (a–d); 50 μm (e–h). C) HEK293 cells were transfected with plasmids expressing Nna1 or the indicated mutants for 40 h and then treated with CHX (50 μg/ml). Levels of Nna1 and its mutants were determined at various times following CHX addition by immunoblotting using an Nna1 antibody. Following protein synthesis, inhibition levels of wild-type Nna1 and Nna1 mutants declined at comparable rates.