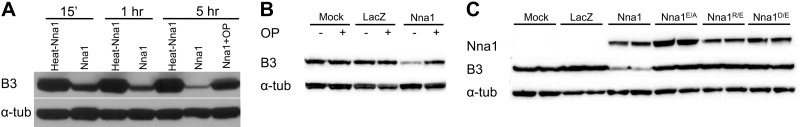
Figure 6.
Nna1 but not Nna1 mutants degrade polyglutamate chain of tubulin. A) Recombinant Nna1 or heat-denatured recombinant Nna1 were incubated for the indicated times with porcine tubulin, and polyglutamylation levels were monitored by immunoblotting with the B3 antibody. Presence of Nna1 (but not heat-denatured Nna1) caused a time-dependent loss of B3-like immunoreactivity, indicating progressive degradation of polyglutamate chains. Addition of 5 mM OP inhibited the activity of Nna1. Levels of α-tubulin served as control for overall tubulin loading and stability and were not affected by Nna1. B) Incubation of tubulin with lysates from HEK293 cells transfected with an Nna1 expression plasmid also reduced B3 immunoreactivity in an OP-inhibitable fashion. C) Catalytic site mutants of Nna1 fail to decrease polyglutamylation level of tubulin. Lysates from HEK293 cells transfected with expression plasmids for Nna1 or the indicated mutants were incubated with tubulin, and B3 immunoreactivity was assessed 5 h later; whereas Nna1 reduced B3 immunoreactivity, the mutants were inactive. Levels of recombinant enzymes were monitored by immunoblotting for Nna1 and were roughly equivalent. Levels of α-tubulin served as loading and integrity control.