Figure 1.
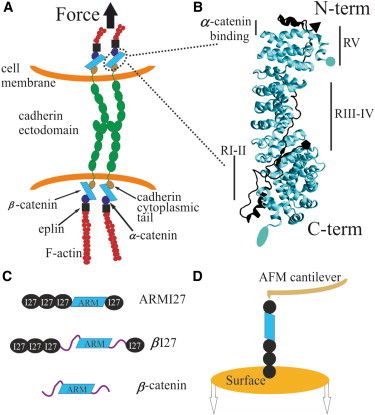
Tertiary and quaternary structure of β-catenin in the adherens junction and the experimental setup for its nanomechanical analysis. (A) Schematic representation of the cadherin adhesion system and possible force applied through the cell-cell adhesion contact. β-catenin is an adaptor protein involved in cell-cell adhesion that can also translocate to the nucleus and act as a transcriptional cofactor when the Wnt signaling pathway is active (4). (B) Crystal structure of the ARM region from β-catenin (lighter structure) complexed with E-cadherin cytoplasmic tail (darker structure). The ARM region is in the middle of the protein and consists of 12 ARM repeats stacked onto one another in a superhelical tertiary structure. β-catenin binds both α-catenin (close to its N-terminal region) and the cadherin cytoplasmic domain (through the ARM region). Circle and pentagon mark the N-termini of β-catenin and cadherin respectively, which are oriented in opposite directions. Ellipse and triangle mark the C-termini of β-catenin and cadherin respectively. (C) We analyzed several fusion proteins comprising four repeats of the titin I27 marker, used for single-molecule identification, flanking either the region of 12 ARM repeats from β-catenin (ARMI27) or the full-length β-catenin (βI27). We also analyzed the full-length β-catenin without the markers. (D) Cartoon representation of the experimental setup: proteins were immobilized on top of a substrate and after their attachment to the cantilever tip of an AFM, these molecules were axially stretched (in the N-C direction).
