Figure 3.
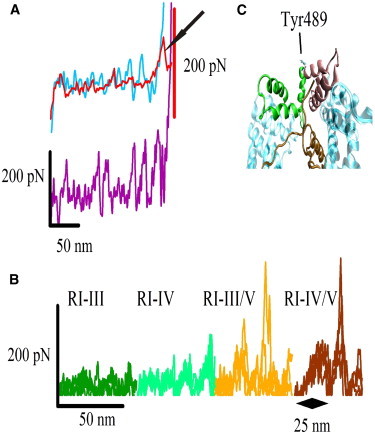
SMD simulations of β-catenin and β-catenin/E-cadherin complex. (A) Force traces for the stretching of the ARM (top) and the ARMHC structures (bottom). An experimental unfolding curve (top, arrow) of the ARM region is superimposed to the in silico curve, showing the similarity between both curves. The structure carrying helix C (ARMHC, bottom) shows slightly higher unfolding force peaks. This evidence goes in line with the increase of high force events observed experimentally when the unstructured termini are present in the structures (Fig. 2B). Thus, β-catenin termini could somehow stabilize the ARM repeats. (B) Superimposition of the four force traces for each β-catenin/E-cadherin complex. The magnitude of the force peaks is higher as longer stretches of E-cadherin are present. A high force peak appears upon breaking the interaction between R1 and RV. (C) Detail of the conformational changes experienced by R8 and R9 ARM repeats resulting in the exposure of the buried Tyr-489.
