Figure 1.
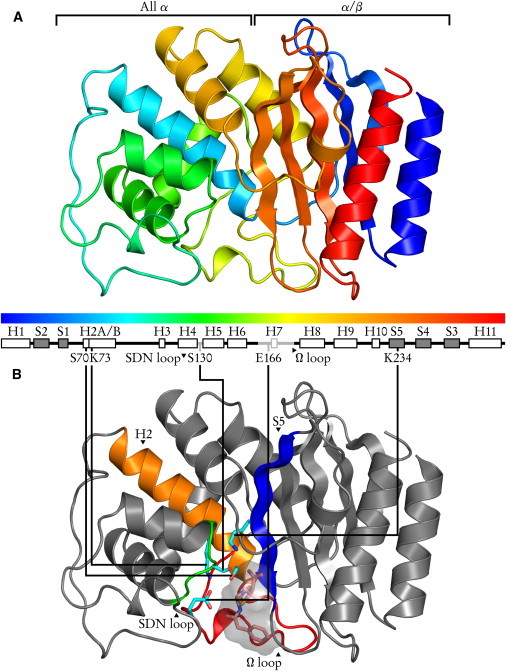
General structure of class A β-lactamases, illustrated by the TEM-1/BZP complex. (A) The N- and C-terminal sequences form the α/β domain, a five-stranded antiparallel sheet bordered by two helices. The all α domain is a bundle of six helices. (B) The active site is at the domain junction. Catalytic residues and substrate are shown as sticks, with CPK-colored atoms: cyan carbons (pink for BZP), red oxygens, blue nitrogens, yellow sulphurs. Substrate volumetric surface is shown in translucent gray. Protein elements forming the catalytic site are colored: blue for the S5 strand bearing catalytic K/R234; orange for H2 providing S70 and K73; green for the SDN loop bearing S130; red for the Ω loop with catalytic E166.
