Abstract
Human EnSC (endometrial-derived stem cell) is an abundant and easily available source for cell replacement therapy. Many investigations have shown the potency of the cells to differentiate into several mesoderm-derived cell lineages, including osteocytes and adipocytes. Here, the potency of EnSC in neural differentiation has been investigated. Flow cytometric analysis showed that they were positive for CD90, CD105, OCT4, CD44 and negative for CD31, CD34, CD133. The characterized cells were induced into neural differentiation by bFGF (basic fibroblast growth factor), PDGF (platelet-derived growth factor) and EGF (epidermal growth factor) signalling molecules, respectively in a sequential protocol, and differentiated cells were analysed for expression of neuronal markers by RT–PCR (reverse transcription–PCR) and immunocytochemistry, including Nestin, GABA (γ-aminobutyric acid), MAP2 (microtubule-associated protein 2), β3-tub (class III β-tubulin) and NF-L (neurofilament-light) at the level of their mRNAs. The expression of MAP2, β3-tub and NF-L proteins in EnSC was confirmed 28 days PT (post-treatment) by immunocytochemistry. In conclusion, EnSC can respond to signalling molecules that are usually used as standards in neural differentiation and can programme neuronal cells, making these cells worth considering as a unique source for cell therapy in neurodegenerative disease.
Keywords: endometrial stem cell, differentiation, neural cell
Abbreviations: β3-tub, class III β-tubulin; bFGF, basic fibroblast growth factor; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; EGF, epidermal growth factor; EnSC, endometrial-derived stem cell; ES, embryonic stem; GABA, γ-aminobutyric acid; GFAP, glial fibrillary acidic protein; HBSS, Hank's balanced salt solution; MAP2, microtubule-associated protein 2; MSC, mesenchymal stem cell; NF-L, neurofilament-light; PDGF, platelet-derived growth factor; PFA, paraformaldehyde; PT, post-treatment; RT–PCR, reverse transcription–PCR; T-PBS, Triton X-100 in PBS
1. Introduction
Stem cells are undifferentiated cells without mature tissue-specific characteristics, and is characterized by the capacity to either proliferate indefinitely (self-renewal) or originate tissue specific committed progenitors or differentiated cells (Joshi and Enver, 2003). Human neurodegenerative disorders, such as stroke, Parkinson's disease and AD (Alzheimer's disease), ALS (amyotrophic lateral sclerosis), epilepsy, trauma and intoxications, are all characterized by neuronal cell loss, associated with consequent loss of function and disabilities (Krabbe et al., 2005). Cell replacement therapy is a potential strategy to cure such diseases. The use of primary fetal brain cells has shown proof-of-principle as a valid approach in small clinical studies (Björklund and Lindvail 2000; Freeman et al., 2000). Various stem cells, such as MSC (mesenchymal stem cell or marrow stromal stem cell), HSC (haemopoietic stem cell), MAPC (multipotent adult progenitor stem cell), UCBSC (umbilical cord blood stem cells) and ES (embryonic stem) cells, have the potency to differentiate into the neural cells (Carpenter et al., 2001; Ortiz-Gonzalez et al., 2004; Cai and Grabel 2007; Lee et al., 2007; Dhara and Stice 2008; Ai et al., 2010; Jiang et al., 2010). However, these cell types are limited by their availability, invasiveness of extraction and in some cases their limited proliferative capacity. Furthermore, MSC from older patients failed to expand in culture, and one might speculate that this is an indication that their effectiveness in general is diminished (Wang et al., 2005; Chua et al., 2009). The human endometrium is a dynamic tissue, which undergoes cycles of growth and regression with each menstrual cycle. Human endometrium contains a low number of EnSC (endometrial-derived stem cell) that seem to belong to the family of the MSC. These cells are engaged in the monthly restructuring and remodelling of a human endometrium (Jabbour et al., 2006; Gargett et al., 2007; Dimitrov et al., 2008). Previous studies have shown the potential differentiation of the EnSC into mesoderm-derived cells, such as chondrogenic and osteoblastic lineages, when cultured in the appropriate induction media (Schwab et al., 2005; Gargett 2006; Kato et al., 2007). Since endometrial stromal cells are easy to isolate, expand rapidly without leading to major ethical and technical problems and produce a higher overall clonogenicity, they have a unique potential as autologous therapeutic agents. Therefore, endometrium may be an alternative source of MSC-like cells for tissue engineering purposes, obtainable with no more morbidity than any other source of stem cells (Gargett 2006; Schwab and Garget, 2007; Patel et al., 2008).
The major aim of our work has been to investigate the ability of EnSC to differentiate in vitro into neuronal cells in response to signalling molecules such as bFGF (basic fibroblast growth factor), PDGF (platelet-derived growth factor) and EGF (epidermal growth factor). Neuronal differentiation was characterized by expression of neural specific markers in both mRNA and protein levels. After differentiation, the majority of EnSC adopted a neuron-like morphology in parallel to the expression of neuronal markers NF-L (neurofilament-light or neurofilament 70 kDa), β3-tub (class III β-tubulin) and MAP2 (microtubule-associated protein 2). The results firmly demonstrate that EnSC can respond to signalling molecules and, in turn, differentiate to neuronal-like cells.
2. Materials and methods
2.1. Biopsy from subjects
Endometrial biopsies were obtained from subjects referred to the hospital for infertility treatment. Exclusion criteria include any endometrial abnormality (polyps, hyperplasia, or cancer) and administration of any hormones, GnRH (gonadotropin-releasing hormone) agonist therapy, or intrauterine device within the last 3 months. A written informed consent form describing the procedures and aims of the study was obtained from each donor, in compliance with regulations concerning the use of human tissues. Endometrial samples were collected from a total of 10 normal ovulating women on cycle days 19–24. The biopsies were obtained from the fundal region of the uterine cavity using an endometrial sampling device.
2.2. Human EnSC isolation
The biopsy tissue was washed in DPBS (Dulbecco's PBS), minced and digested in HBSS (Hank's balanced salt solution; Gibco) containing Hepes (25 mM), collagenase A (1 mg/ml, Gibco) for 30–45 min at 37°C with agitation. Resultant dispersed cell solutions were passed through a 70 μM sieve (BD Biosciences) to remove glandular epithelial components. The cells were centrifuged, and the mononuclear cells separated by Ficoll were washed in PBS. These cells were cultured in DMEM (Dulbecco's modified Eagle's medium)/F12 medium containing 10% FBS, 1% antibiotic penicillin/streptomycin and 1% glutamine and incubated at 37°C in 5% CO2 in air (Chan et al., 2004).
2.3. Phenotypic characterization
Three passages after isolation, the cells were characterized by flow cytometry for surface markers. Cells were washed with HBSS+2% BSA twice and incubated with the specific antibody at concentrations recommended by their respective suppliers. After 20 min incubation they were analysed by flow cytometry. The antibodies used were: FITC-conjugated anti-CD90, PE anti-human CD105, FITC-conjugated anti-CD44 (mesenchymal markers), FITC-conjugated anti-CD34 and FITC-conjugated anti-CD133 (haemopoietic marker), PE anti-human CD31 (endothelial marker) and FITC-conjugated mouse IgG1, PE-conjugated mouse IgG1 was used for negative control, all from Santa Cruz. For intracellular staining by the antibodies without any conjugate, cells were washed twice in Hank's solution with 2% BSA and fixed with 4% (w/v) PFA (paraformaldehyde) for 1 h. Subsequently, cells were washed twice in 0.5% Tween 20 and 0.1% T-PBS (Triton X-100 in PBS). Primary antibodies were added to T-PBS at the concentrations recommended by the manufacturer. After 30 min incubation, the cells were washed twice in T-PBS. Corresponding secondary antibodies with fluorescent conjugate were subsequently diluted in T-PBS at the concentrations recommended by the manufacturer. Following incubation for 20 min, the cells were analysed using flow cytometry (Partec). The intracellular antibodies used were OCT4 (clone Y182, hTert: Abcam). Cells analysed by intracellular staining were at passage 12.
2.4. Adipogenic differentiation
EnSC were seeded at 2×104 cells/ml in 24 well dishes with 0.5 ml media per well. When the cells had reached 100% confluence, they were transferred to adipogenic induction media [insulin (10 g/ml), dexamethasone (1 M), indomethacin (200 M) and isobutylmethylxanthine (0.5 mM) (Schwab et al., 2008)] and cultured for 10 days, with media changes every 3–4 days. Control cells were cultured in completed DMEM media. Cells are subsequently stained with Oil Red O (Sigma) and visualized under fluorescent microscopy.
2.5. Osteogenic differentiation
EnSC were seeded at 2×104 cells/ml with 0.5 ml complete DMEM per well. After the cells had adhered overnight, the medium was changed to osteogenic induction medium (10−7 M dexamethasone, 50 μg/ml l-ascorbic acid-2-phosphate and 10 mM β-glycerophosphate). Cultures were cultured for 28 days with medium changes every 3–4 days. Control cells were cultured in complete DMEM. Cells were stained with Alizarin Red (Sigma) and visualized by microscopy.
2.6. Chemical induction for neuronal differentiation
Tissue culture-treated chamber slides (Nalge Nunc) were coated with 0.01% fibronectin (Sigma) for 60 min at room temperature, washed with deionized water and allowed to dry. Cells were seeded into the chamber slide at 5000/cm2 with basic medium at passage 3. After 24 h, the basic medium was replaced by neural differentiation media. Cells were maintained in pre-induction media containing DMEM/F12 (Invitrogen), 1% penicillin/streptomycin, 2 mM glutamax (Igbo), 1×N2 supplement (Invitrogen) and 100 ng/ml bFGF (R&D Systems Inc.) for 4 days. The pre-induction media were removed, and the cells were washed with PBS (Gibco-BRL) before being transferred to neuronal induction media composed of DMEM/F12+10% FBS, 10 ng/ml bFGF, 20 ng/ml hEGF (human epidermal growth factor; R&D Systems Inc.), 10 ng/ml PDGF-AA (R&D Systems Inc.) for 5 days. The media were removed, and new induction media were added containing 10 ng/ml bFGF+10 ng/ml PDGF-AA, excluding EGF for 7 days totalling 16 days. Cells were cultured on fibronectin-coated coverslips, and full medium changes were made every 2–3 days with fresh growth factors.
2.7. Morphological observation
Cells were observed under a phase-contrast microscope to evaluate their overall appearance. Microphotographs were taken with ×10 objective (TS-100 Nikon).
2.8. Immunocytochemical analysis
Day 12 PT (post-treatment) cells were fixed with 4% PFA (Sigma–Aldrich) for 30 min at room temperature. After permeabilization with 0.2% Triton X-100 (Sigma–Aldrich) for 10 min, the cells were blocked with goat serum and incubated sequentially overnight with primary antibody MAP2 (mouse monoclonal anti-human; Abcam), anti-β3-tub (mouse monoclonal anti-human; Abcam), NF-L (mouse monoclonal anti-human; Abcam) and Olig1 (mouse monoclonal anti-human; Abcam) at a 1:500 dilution at 4°C, and for 4 h with secondary antibody (rabbit anti mouse IgG-FITC, at a 1:200 dilution; Abcam) at 37°C. Between each step, slides were washed with PBS and nuclei stained with DAPI (4′,6-diamidino-2-phenylindole; Sigma). Cells were examined by fluorescence microscope (Olympus BX51).
2.9. RNA extraction and analysis
RT–PCR (reverse transcription–PCR) analysis of the changing expression of various cell-type markers during the differentiation from EnSC into a neural cell lineage was performed on mRNA collected from differentiated cells after 7 and 12 days induction. Total RNA was extracted by TRIzol® Reagent (Invitrogen). Subsequently, 5 mg total RNA was converted into cDNA by using MMLV (Moloney-murine-leukaemia virus) Superscript II reverse transcriptase (Promega) and random primers (Oligo dT18; Promega). The forward and reverse primers used are listed in Table 1. Each RT–PCR analysis was done on three samples.
Table 1. Primers used in RT–PCR for neural markers.
| Gene | Accession no. | Primer sequence (5′–3′) | Size (bp) | Annealing (°C) |
|---|---|---|---|---|
| Nestin | NM_006617 | F:AGCAGCACTCTTAACTTACG | 259 | 55 |
| R:CTGACTTAGCCTATGAGATGG | ||||
| Vimentin | NM_003380 | F:GCTCAGATTCAGGAACAG | 621 | 55 |
| R:GCAGGTCTTGGTATTCAC | ||||
| Map2 | NM_002374 | F:TGAAGAATGGCAGATGAAC | 214 | 56 |
| R:AGAAGGAGGCAGATTAGC | ||||
| Olig1 | NM_138983 | F:TAACCAGGCGTCTCACAG | 347 | 55 |
| R:ATTCGGCTACTACCAACAAC | ||||
| GFAP | NM_002055 | F:GAATGAGGAGGAAGGAGAG | 282 | 57 |
| R:CCAGGAGTTCAAGGTCAG | ||||
| GABA | NM_001182 | F:TAACAACGAGCCAATAGC | 475 | 55 |
| R:GACAGCCACACTAATGAG | ||||
| β-actin | NM_001101 | F:CGTGACATTAAGGAGAAG | 202 | 56 |
| R:TGATGGAGTTGAAGGTAG |
3. Results
3.1. Characterization of isolated human EnSC
After plating for 24 h, some adherent MSC appeared in the flask, and the cells were heterogeneous in appearance. Approximately 10 days later, these cells developed into many clusters, and could be used for sub-culturing. After 3–4 passages, human EnSC became relatively homogeneous in appearance, being relatively elongated or spindle-shaped (Figure 1).
Figure 1. Phase-contrast photomicrograph showing morphological characteristics of passage 3 EnSC in culture.
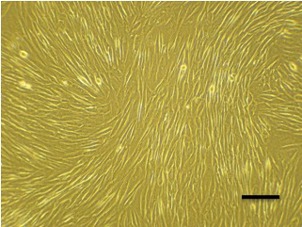
Scale bar: 100 μm.
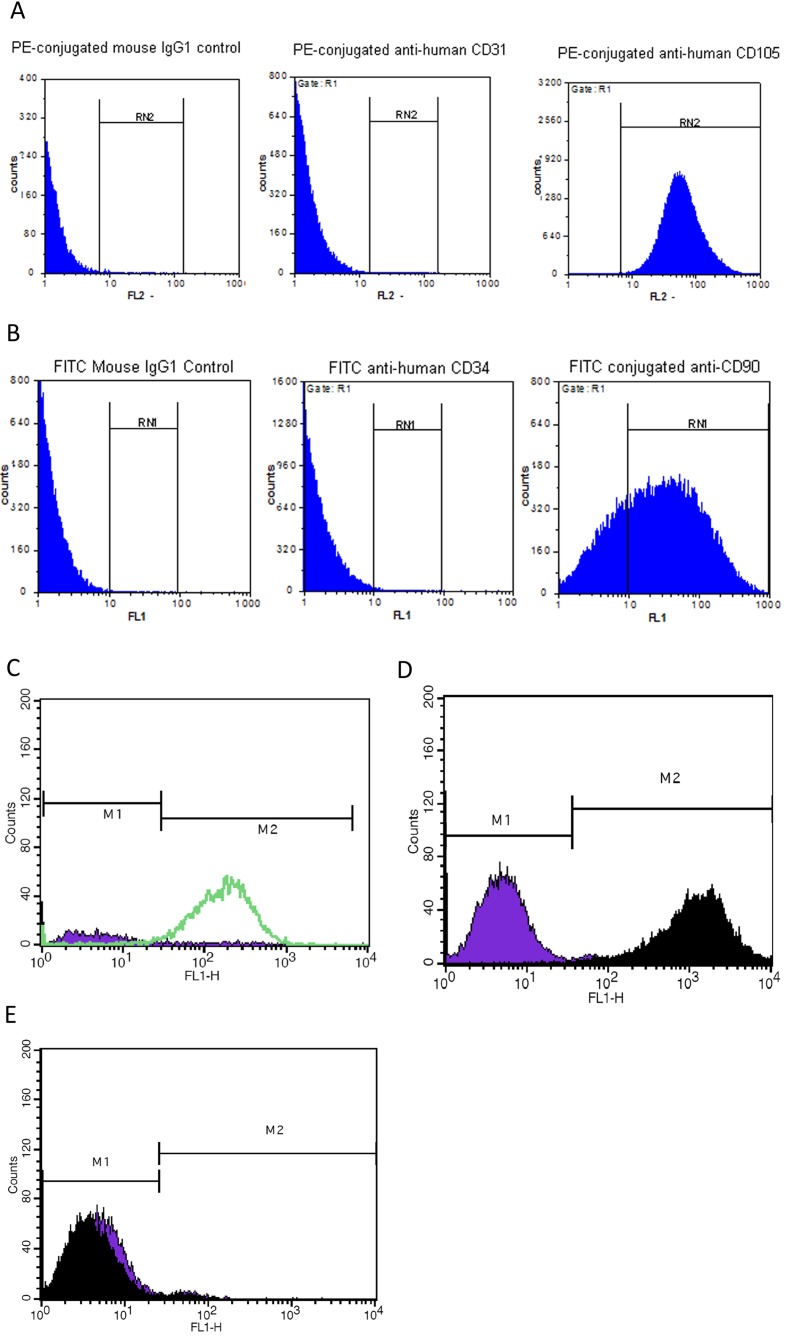
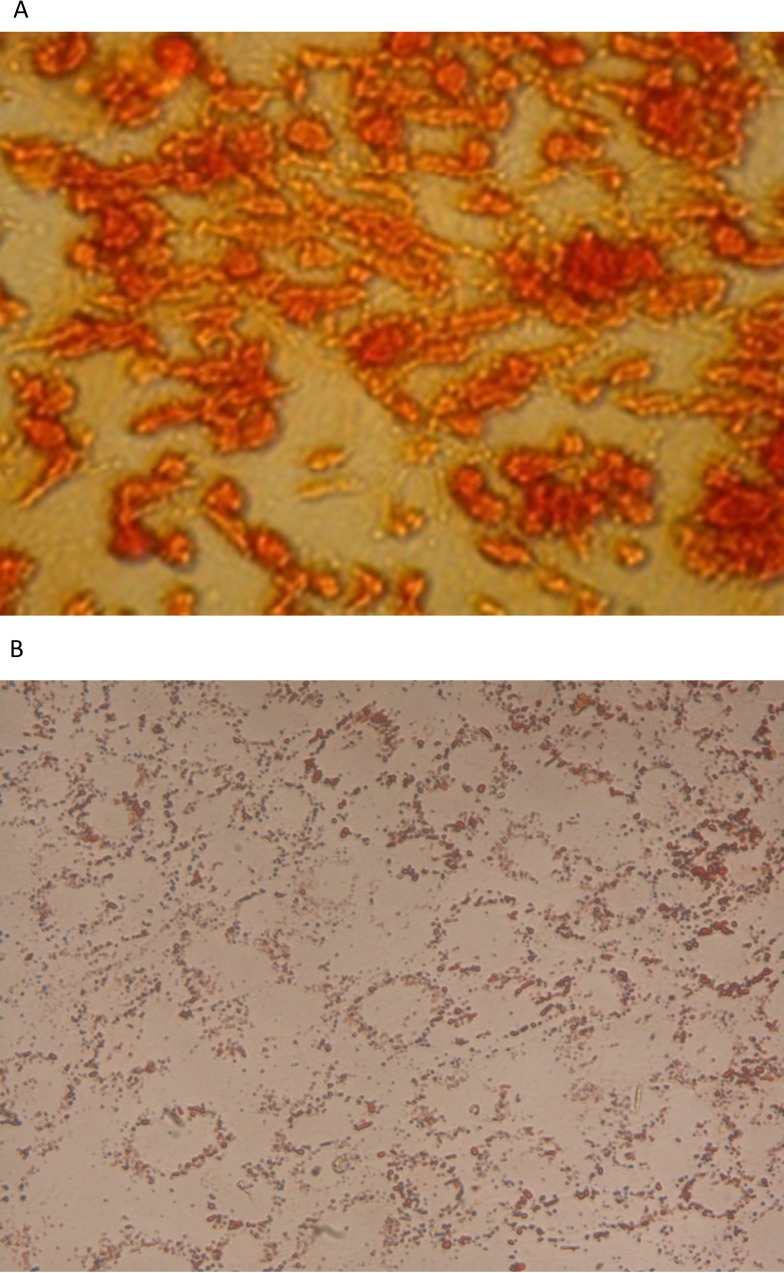
The immunophenotype was based on the flow cytometric analysis of a subset of ES cell marker (OCT4), MSC markers (CD90, CD105 and CD44), haemopoietic markers (CD34 and CD133) and endothelial marker (CD31). The analysis showed that they were positive for CD90, CD105 and OCT4 and negative for CD31 and CD34 (Figure 2). Given the phenotypic, morphological, proliferative characteristics of the EnSC, we determined whether these cells were capable of differentiating into various lineages as can other stem cell types. Differentiation into osteocytes and adipocytes was demonstrated by culturing of EnSC using standard commercially available culture reagents and methodologies. Subsequent to treatment with differentiation inducing protocols (Figure 3), EnSC could differentiate into osteocytes and adipocytes.
Figure 2. Flow cytometric analysis of isolated EnSC for mesenchymal stem cell markers (CD90, CD105 and CD44), haemopoietic marker (CD34 and CD133), endothelial marker (CD31) and ES cell marker (OCT4).
As shown the isolated cells are positive for CD90, CD105, CD44 and OCT4 and are negative for CD31, CD34 and CD133.
Figure 3. EnSC differentiate into osteocytes (A) staining with Alizarin Red and adipocytes (B) staining with Oil Red O (×400 magnification).
3.2. Analysis of neural differentiation
3.2.1. Morphological differentiation
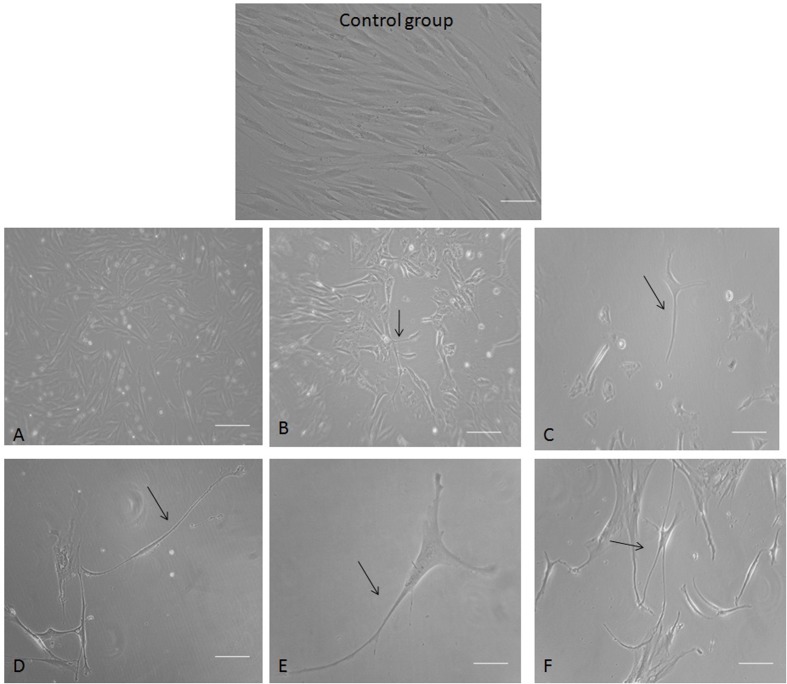
To induce neural differentiation of human EnSC in long-term cultures, the cells were cultured in the classical serum-free medium (DMEM/F12 supplemented with N2) and induced by bFGF, PDGF and EGF signalling molecules, which classically can lead to neural differentiation. To identify neuro-glial differentiation, the differentiating cells were observed daily by phase-contrast microscopy (Figure 4). After 7 days exposure to neural inducing signalling molecules, changes in the shape of most cells were observed (Figures 4A–4C). Some short neurite-like extensions were obvious after 7 days PT, and easily recognizable and fully developed after 12 days PT (Figures 4D–4F). Initially, cytoplasm in EnSC retracted towards the nucleus, forming a contracted multipolar cell body and leaving some process-like extensions peripherally. The cell bodies became increasingly pyramidal or spherical, and refractile, exhibiting a typical neuronal perikaryal appearance. The cell process became longer and more evident (arrows show branching processes in all of Figures).
Figure 4. Human EnSC 7 and 12 days PT by neural inducing signalling molecules.
(A–C) Neuronal-like cells derived from EnSC 7 days PT. (D–F) Differentiated cells 12 days PT with a clear morphological neuronal shape (consisting of b-fibre-bearing cells with features typical of cultured neurons, that is sharply defined, phase-bright bodies, and thin, long, often branching processes (arrows show branching processes). Scale bar: 100 μm.
3.2.2. Immunocytochemical analysis
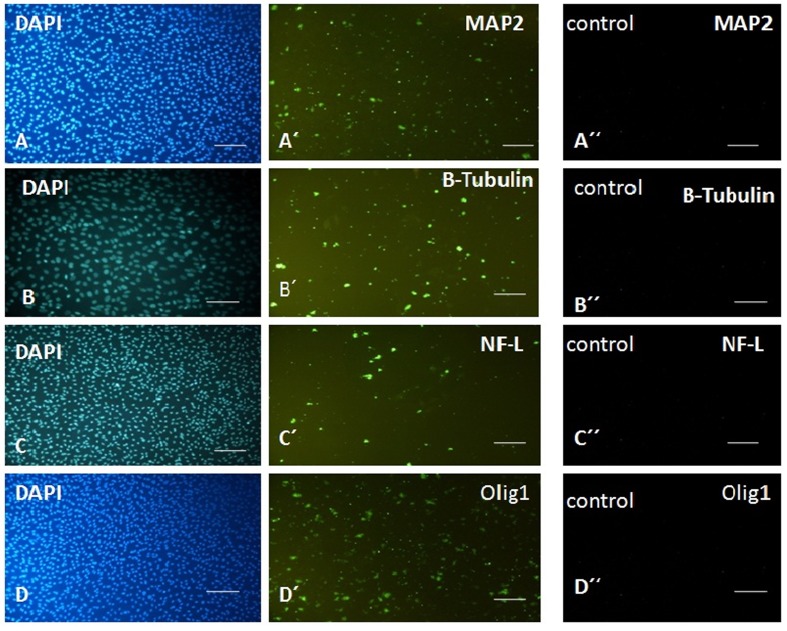
To correlate the morphological changes with neuronal differentiation, we analysed the expression of neuron-specific markers in differentiated neuronal-like cells by immunocytochemistry. Neuronal cells are classically characterized by the expression of cytoskeletal proteins that include NF-L, β3-tub, MAP2 and olig1, which are expressed in mature neurons. Figure 4 shows the expression of these mature markers in neuronal-like cell differentiated from EnSC only after induction (Figures 5A′, 5B′, 5C′ and 5D′) by signalling molecules, while they were not expressed in EnSC before neuronal differentiation (Figures 5A″, 5B″, 5C″ and 5D″). Thus EnSC can respond to the signalling molecules, differentiate into neurons and express the mature neuronal markers.
Figure 5. Immunocytochemical analysis for expression of MAP2, β3-tub, NF-L and Olig1 as markers of mature neurons and oligodendrocyte in EnSC before neuronal induction (A′′, B′′, C′′and D′′) and 12 days PT by neuronal inducing signalling molecules (A′, B′ and C′).
Nuclei were stained with DAPI (A, B and C). Scale bar: 100 μm.
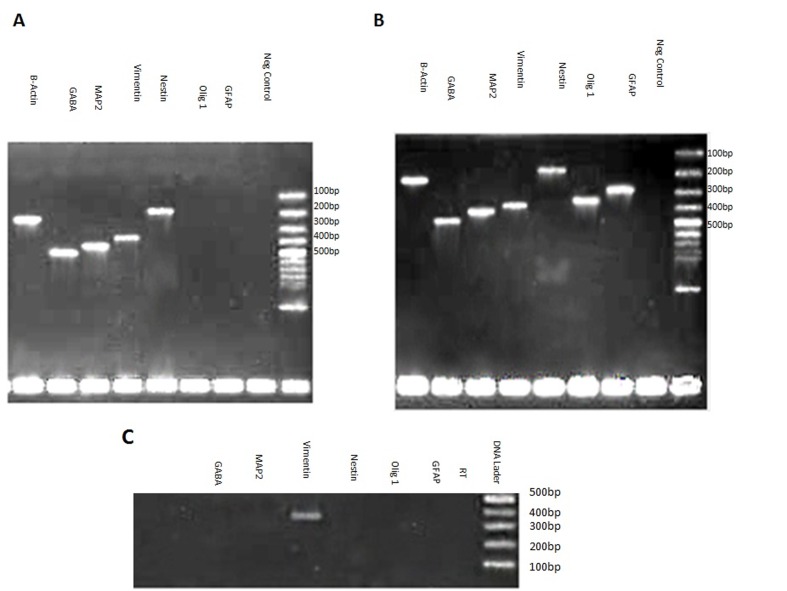
3.2.3. RT–PCR analysis
To investigate the expression of neural markers at the level of mRNA, RT–PCR was carried out (Figure 6). The expression of MAP2, which has already been shown at the level of protein (Figure 5A′), was also confirmed at the mRNA level at both 7 and 12 days PT. However, the expression of GABA (γ-aminobutyric acid) and Nestin transcripts, which were not detected in EnSC cells (Figure 6C), was detected after differentiation in EnSC-derived neuronal-like cells at both 7 and 12 days PT (Figures 6A and 6B), implying that they were promoting neuronal programming in differentiated cells. In a control group, expression of vimentin as in the treatment group was detected. To investigate the derivation of astrocyte and oligodendrocyte from EnSC upon differentiation using the abovementioned protocol, the expression of GFAP (glial fibrillary acidic protein) and Olig1 as specific markers of astrocytes and oligodendrocyte respectively was analysed by RT–PCR. Expression of these two markers was not detected 7 days PT, while they were clearly expressed at 12 days. The result, at least in the level of mRNA, implies that these cells can adopt a neuronal differentiation pathway in a short time compared with data on other adult stem cells (Bossolasco et al., 2005; Chua et al., 2009), and with more time differentiation into other neural cell lineages will occur.
Figure 6. Neuro-glial-related gene expression analysis of EnSC 7 days PT (A) and 12 days PT (B) treatment and control (C) for the using RT–PCR.
β-Actin was used as internal standard.
4. Discussion
The EnSC present themselves as a new source of MSC (Meng et al., 2007; Gargett et al., 2009). In the present study, we isolated, characterized and differentiated EnSC to neural fate using signalling molecules, such as bFGF, PDGF and EGF and without the use of RA (retinoic acid). We also showed that these cells can differentiate into glial cells. The EnSC in response to the signalling molecules can programme them to become neurons in 7 days and to glial cell lineage after an additional 5 days. The EnSC-derived neuron-like cells could express neuronal markers, including NF-L, MAP2 and β3-tub, associated with distinct morphological modifications. The data support the possibility of the wider application of EnSC in cell therapy of neurodegenerative diseases in which the surroundings of non-neuronal cells must be considered. EnSC displaying excellent pluripotency potential exist in the basal layer of the endometrium of menopausal women (Gargett et al., 2007). Previous studies concerning long-term follow-up of animals treated with endometrial regenerative cells, with the karyotypic normality of these cells after extended passage (68 doublings), confirmed their lack of tumorigenicity (Deschaseaux and Charbord 2000; Shi and Gronthos 2003; Buhring et al., 2007; Montzka et al., 2009).
Trans-differentiation of MSC into neurons has aroused great interest since two pioneering works in 2000 (Sanchez-Ramos et al., 2000; Woodbury et al., 2000). The former authors reported having induced rat and human MSC to differentiate into neuronal-like cells by treating MSC with neuronal induction media. Following studies have attempted to demonstrate this neuronal differentiation potential of MSC in vitro (Deng et al., 2006; Bossolasco et al., 2005; Tropel et al., 2006; Lei et al., 2007; Jiang et al., 2010), and in vivo by transplantation (Azizi et al., 1998; Brazelton et al., 2000; Munoz-Elias et al., 2004). However, these findings and interpretations have been challenged.
Meng et al. (2007) and Wolff et al. (2010) used endometrial stem cell derived from menstrual blood and endometrial stem cell respectively for neural differentiation. They used RA for neural differentiation and did not detect glial cells from endometrial stem cell. In our study, the data clearly demonstrate EnSC differentiating into a neuronal phenotype in vitro without RA. We have shown that after 7 days of induction, neuronal-like phenotypes appeared and these cells were elongated by day 12 PT. Besides the morphological evidence, we have also demonstrated that the neuronal-like phenotypes derived from EnSC express cell surface markers specific to neuron cells. Moreover, the expression mRNA levels of Olig1 and GFAP did not detect in 7 days PT, while it was obvious in 12 days PT, implying that the short-time protocol is enough to derive a neuron from EnSC.
It can be concluded that EnSC are more convenient than other sources of stem cells in the treatment of neuronal disorders because of the following properties. First, obtaining bone marrow stem cells in the clinic is invasive, because of the requirement for anaesthesia, while EnSC can be obtained by a simple, safe and painless procedure such as Pap smears, in contrast with bone marrow aspiration. Secondly, bone marrow MSC are not perfect seeding cells for the elderly patients since these cells lose their differentiation capacity significantly with increasing donor age (Meng et al., 2007).
In the past few years, research on the stem cells has exploded as a means to build probable therapies for the treatment of inoperable neurodegenerative diseases. Despite the promising results, significant restraints hinder the use of different sources of stem cells for transplantation in humans. Besides ethical concerns, the viability, purity, carcinogenic potency and final destiny of the cells have not been completely defined. A highly promising source of relatively abundant and accessible, active, pluripotent adult stem cells is afforded by the human endometrium. Consequently, we speculate that endometrial adult stem cells can differentiate into neural cells when they are exposed to specific signalling molecules. EnSC is an attractive alternative candidate for nervous system regeneration, because they exhibit several important and potential advantages over other stem cells.
Footnotes
This work was supported by the Tehran University of Medical Sciences Research [grant number 88-02-85-8584] and the Iranian Council of Stem Cell Technology.
Author contribution
Zahra Taherian Mobarakeh did the cell culture and flow cytometry. Jafar Ai and Somayeh Ebrahimi Barough did the cell culture and neural differentiation. Farzad Yazdani Biuocki did the adipocyte differentiation. Seyed Mahdi Rezayat Sorkhabadi did the osteoblast differentiation. Mohammad Vasei did the RT-PCR. Zeinab Ghanbari prepared of endometrial biopsy. Abbas Noroozi Javidan and Mohammad Massumi did the immunocytochemistry. Seyed AbdolReza Martazavi-Tabatabaei helped with the cell culture and flow cytometry analysis.
References
- Ai J, Noroozi A, Mehrabani D. The possibility of differentiation of human endometrial stem cells into neural cells. Iran Red Crescent Med J. 2010;12:328–31. [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–13. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–44. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Lambertenghi G. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005;193:312–25. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FMV, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Buhring HJ, Battula V, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann NY Acad Sci. 2007;1106:262–71. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- Cai C, Grabel L. Directing the differentiation of embryonic stem cells to neural stem cells. Dev Dyn. 2007;236:3255–66. doi: 10.1002/dvdy.21306. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–97. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- Chua SJ, Bielecki R, Wong CJ, Yamanaka N, Rogers IM, Casper RF. Neural progenitors, neurons and oligodendrocytes from human umbilical cord blood cells in a serum- free, feeder free cell culture. Biochem Biophys Res Commun. 2009;379:217–21. doi: 10.1016/j.bbrc.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Deng J, Petersen E, Steindler D, Jorgensen M, Laywell E. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–64. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- Deschaseaux F, Charbord P. Human marrow stromal precursors are alpha 1 integrin subunit-positive. J Cell Physiol. 2000;184:319–25. doi: 10.1002/1097-4652(200009)184:3<319::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J Cell Biochem. 2008;105:633–40. doi: 10.1002/jcb.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135:551–8. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- Freeman TB, Cicchetti F, Hauser RA, Deacon TW, Li XJ, Hersch SM. Transplanted fetal striatum in Huntington's disease: phenotypic development and lack of pathology. Proc Natl Acad Sci USA. 2000;97:13877–82. doi: 10.1073/pnas.97.25.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE. Identification and characterization of human endometrial stem/progenitor cells. Aust N Z J Obstet Gynaecol. 2006;46:250–3. doi: 10.1111/j.1479-828X.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Chan RW, Schwab KE. Endometrial stem cells. Curr Opin Obstet Gynecol. 2007;19:377–83. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- Gargett C, Schwab K, Zillwood R, Nguyen H, Wu Di. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Jiang J, Lv Z, Gu Y, Li J, Xu L, Xu W. Adult rat mesenchymal stem cells differentiate into neuronal-like phenotype and express a variety of neuro-regulatory molecules in vitro. Neurosci Res. 2010;66:46–52. doi: 10.1016/j.neures.2009.09.1711. [DOI] [PubMed] [Google Scholar]
- Joshi C, Enver T. Molecular complexities of stem cells. Curr Opin Hematol. 2003;10:220–228. doi: 10.1097/00062752-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Kato K, Yoshimoto M, Kato K, Adachi S, Yamayoshi A, Arima T. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22:1214–23. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- Krabbe C, Zimmer J, Meyer M. Neural transdifferentiation of mesenchymal stem cells – a critical review. Act Pathol Microbiol Immunol Scand. 2005;113:831–44. doi: 10.1111/j.1600-0463.2005.apm_3061.x. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–75. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lei Z, Yongda L, Jun M, Yingyu S, Shaoju Z, Xinwen Z, Mingxue Z. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int. 2007;31:916–923. doi: 10.1016/j.cellbi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Meng X, Ichim T, Zhong J, Rogers A, Yin Z, Jackson J. Endometrial regenerative cells: a novel stem cell population. J of Trans Medic. 2007;5:1–10. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montzka K, Lassonczyk N, Tschöke B, Neuss S, Führmann T, Franzen R. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: misleading marker gene expression. BMC Neurosci. 2009;10:16. doi: 10.1186/1471-2202-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–95. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Gonzalez XR, Keene CD, Verfaillie CM, Low WC. Neural induction of adult bone marrow and umbilical cord stem cells. Curr Neurovasc Res. 2004;1:207–13. doi: 10.2174/1567202043362342. [DOI] [PubMed] [Google Scholar]
- Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17:303–11. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–56. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84:1124–30. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23:934–43. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE. Coexpression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Tropel P, Platet N, Platel J, Albrieux M, Benabid A, Bergera F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–76. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- Wang Y, Deng Z, Lai X, Tu W. Differentiation of human bone marrow stromal cells into neural-like cells induced by sodium ferulate in vitro. Cell Mol Immunol. 2005;2:225–9. [PubMed] [Google Scholar]
- Wolff E, Gao X, Yao K, Andrews Z, Du H, Elsworth J, Taylor H. Endometrial stem cell transplantation restores dopamine production in a Parkinson disease model. J Cell Mol Med. 2010;15:747–55. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]