Abstract
Mesenchymal stem cells (MSCs) are non-hematopoietic, pluripotent cells that give rise to stromal cells in the marrow. MSCs have been shown to be immunosuppressive and have become an attractive therapeutic option for the modulation of undesired immune responses. Currently, ex vivo expanded human (h)MSCs are being utilized in clinical trials both in the USA and in Europe to treat a variety of immune disorders. hMSCs need to be harvested, isolated and expanded in culture. This necessary expansion may also result in decrease or loss of the immunomodulatory potential of hMSCs. Ideally, the intrinsic immunomodulatory activity (potency) of an hMSC preparation should be assessed prior to its administration. The goal of the experiments described here was to develop a simple potency assay for the immunomodulatory properties of hMSCs. The immunosuppressive activity of hMSCs conditioned media was tested in enzyme-linked immunosorbent spot assays (ELISpot) and the immunosuppressive activity of the conditioned media was correlated with the concentration of several cytokines present in these conditioned media. The concentration of prostaglandin E2 in the media correlated with their immunosuppressive activity. The concentration of the other cytokines measured did not correlate with the immunosuppressive activity of the media. The dose-response effect could be replicated by adding PGE2 to ELISpot assays. Furthermore, the immunosuppressive activity of the conditioned media was inhibitable by a neutralizing anti-PGE2 antibody. These data suggest that measurement of PGE2 in media conditioned by hMSCs exposed to inflammatory stimuli could be used as a surrogate measure of their immunosuppressive capacity. These findings need to be confirmed in vitro using different assays of immune function and validated in vivo to determine the level of correlation of these data with efficacy in pre-clinical models of immune disorders.
Keywords: Mesenchymal stem cell, immunosuppression, prostaglandin E2, cellular therapy, cell culture
Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic, pluripotent cells that give rise to stromal cells in the marrow. These cells produce cytokines, chemokines and extracellular matrix proteins involved in hematopoietic stem cell (HSC) homing and proliferation [1]. In addition to supporting in vitro HSC survival and proliferation and in vivo HSC engraftment, MSCs have been shown to interact with immune effector cells. MSCs stimulated with allogeneic CD14+ mononuclear cells (MNCs) can decrease T-cell activation and its associated interferon-gamma (IFN-γ) production [2,3]. Thus, MSCs are an attractive therapeutic option for the modulation of undesired immune responses [4,5].
Currently, ex vivo expanded human (h)MSCs are being utilized in clinical trials both in the USA and in Europe to treat a variety of immune disorders [6,7]. For this purpose, MSCs need to be harvested from the donor, and expanded in culture, sometimes considerably, in order to obtain sufficient numbers of cells to infuse into the affected patients [8]. These necessary cell isolation and expansion steps add a lag time of several weeks between the initial harvest of the bone marrow, and the infusion of the cells. This necessary expansion and time in culture may also result in the decrease or loss of the immunomodulatory potential of MSCs. Ideally, the intrinsic immunomodulatory activity (potency) of an hMSC preparation should be assessed prior to its administration.
The Federal Drug Administration (FDA)’s code of federal regulations (CFR) title 21 part 61 (21 CFR 61) identifies key elements (safety, sterility, purity, identity and potency) necessary for successful development of a cellular product. Currently, there are well-defined and relatively simple assays to assess the sterility, purity and identity of hMSCs as a cellular therapeutic, but potency assays for their immunosuppressive and paracrine functions are either not well defined or require complex processes involving multiple-day co-cultures with other cell preparations.
In 21 CFR 600.3(s), “Potency is interpreted to mean the specific ability or capacity of the product to effect a given result” and in 21 CFR 610.10, FDA requires that “tests for potency consist of either in vitro or in vivo tests, or both, which have been specifically designed for each product as to indicate its potency”.
For this particular application of hMSCs, the desired effect is effective modulation of immune cell responses whether triggered by alloantigens such as in graft-versus-host disease, or aberrant responses to other cellular and extracellular components such as in multiple sclerosis, rheumatoid arthritis, asthma, etc.
The development and validation of a simple, standardized assay to measure the immunomodulatory properties of hMSC preparations would contribute to the standardization of MSC-based cellular therapies.
The goal of the experiments described here was to develop a simple potency assay for the immunomodulatory properties of hMSCs.
Materials and methods
Isolation of hMSCs
Eight human MSC (hMSC) preparations were derived from bone marrow aspirated from the iliac crest of normal healthy human donors at the Hematopoietic Stem Cell Core Facility at Case Western Reserve University after informed consent obtained under the terms of an Internal Review Board-approved protocol.
The procedures for establishing human bone marrow-derived hMSC cultures followed previously published methods [9,10]. Briefly, bone marrow aspirates were washed with complete hMSC medium consisting in low glucose Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum from a selected lot (Gibco, Grand Island, NY) [10] (DMEM-LG + 10% FBS) and subjected to a pre-formed Percoll (Sigma Chemical Co., St. Louis, MO) density gradient to isolate mononuclear cells. Serum lot selection is a standard procedure performed prior to purchasing a new shipment of serum; all these experiments were conducted with serum from a single lot. The mononuclear cells were washed with complete medium and seeded at a density of 1.8 x 105 cells/cm2 to establish primary cultures of human bone marrow-derived hMSCs. All cell-culture was done at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Subculturing
In order to keep their growth at an exponential rate and prevent spontaneous differentiation or loss of differentiation potential, hMSCs must be subcultured before the cells become confluent [9,11]. Typically, they are passaged when the cultures were 80-90% confluent. Primary cultures were usually subcultured around day 14 ± 3 days Subsequently, the cells were subcultured approximately every 7 ± 2 days. Cells were subcultured by trypsinization, counted, and reseeded at a density of 4.5 x 103 cells per cm2.
Stimulation of hMSCs
Third passage cells were seeded into 6 well plates, at a density 15 x 103 cells/cm2 in complete hMSC medium and the plates incubated overnight; the medium was removed and complete PBMC medium consisting in Roswell Park Memorial Institute (RPMI)-1640 (Invitrogen) supplemented with 10% heat-inactivated FBS (Sigma), further supplemented with interleukin-1 beta (IL-1β) (Peprotech, Rocky Hill, NJ) (5 pg/ml), tumor necrosis factor-alpha (TNF-α) (Peprotech) (25 ng/ml) or both IL-1β and TNF-α, was added to the wells. Control wells received only complete PBMC medium. Another set of control wells were incubated in complete PBMC medium without hMSCs, but still received IL-1β, TNF-α or both IL-1β and TNF-α. After a 24-hour incubation period, the conditioned media were collected into 2-ml microcentrifuge tubes and centrifugated for 10 minutes at 13,000 rpm to remove any remaining cells. The supernatants were then transferred to clean microcentrifuge tubes and either used fresh or frozen at -80°C for later use.
In a separate series of experiments, serum-free medium consisting of RPMI supplemented with 1% ITS+ Premix (BD biosciences, Franklin Lakes, NJ) was utilized as the base medium instead if complete PBMC medium.
Isolation of PBMCs
Human PBMCs were isolated from peripheral blood from normal healthy human donors after informed consent obtained under the terms of an Internal Review Board-approved protocol at the Hematopoietic Stem Cell Core Facility at Case Western Reserve University.
The blood was carefully layered on top of Ficoll (GE, Piscataway, NJ) and the tubes centrifuged at 800 x g for 30 minutes without brake. A sterile plastic pipette was used to aspirate the PBMCs and transfer them into a new 50 ml conical tube. The PBMCs were washed twice with and resuspended in complete PBMC medium.
ELISpot
Ninety-six-well ELISpot plates (Millipore MultiS-creen HTS®IP) were coated with anti-human interferon-gamma (IFN-γ) antibody (Pierce, Rockford, IL); 100 μl of antibody solution (4 μg/ml in PBS) were added to each of the 96 wells of the plate and incubated overnight in the refrigerator. The plates were then washed with PBS and blocked at 37°C for 2 hours with complete RPMI. The wells then received either 150 μl of complete RPMI (control wells) or 150 μl of either 106 cells/ml hMSC suspension or hMSC-conditioned medium (experimental wells). Then 25 μl of complete RPMI were added to the negative control wells and 25 μl of phytohemagglutinin (PHA) solution (40 μg/ml in complete RPMI) were added to experimental and positive control wells. A 25-μl aliquot of PBMC suspension (6.0 x 106 cells/ml) was finally added to each well and the plate was incubated for 24 hours at 37°C. After the incubation, the plate was washed with PBS + 0.05% Tween; biotinylated anti-IFN-γ antibody (Pierce) (2 μg/ml in PBS + 0.05% Tween + 1% BSA) was added and the plate incubated at 37°C for 2 hours. After washing the plate with PBS + 0.05% Tween; Streptavidin-HRP (Dako) diluted 1:1,000 in PBS + 0.05% Tween + 1% BSA was added and the plate incubated for 1 hour. After 3 washes with PBS + 0.05% Tween, followed by 4 washes with PBS, the IFN-γ-positive spots were developed with 3-amino-9-ethyl carbazole (AEC) (Pierce). The reaction was then stopped with tap water and the plates were allowed to dry in the dark. The plates were analyzed with a computer-assisted ELISpot analyzer (Cellular Technology Inc., Cleveland, OH). Percent inhibition was obtained by direct comparison to the corresponding positive control indicated above.
Cytokine assays
The content of prostaglandin E2 (PGE2), transforming growth factor beta 1 (TGF-β1), hepatocyte growth factor (HGF), interleukin 10 (IL-10), and vascular endothelial growth factor (VGEF) in the conditioned media was measured using Multi-array® kits (Meso Scale Discovery; Gaithersburg, MD and Cayman Chemical; Ann Arbor, MI) according to the manufacturer’s instructions. The plates were analyzed with a SEC-TOR Imager (SI2400; Meso Scale Discovery).
Immunosuppressive activity of exogenous PGE2
Series of test media (both Complete RPMI and ITS-supplemented RPMI) with increasing concentrations of PGE2 (Cayman, Ann Arbor, MI) were prepared. The immunosuppressive activity of the PGE2 containing media was tested in IFN-γ ELISpot.
Blocking PGE2 activity with antibodies
Aliquots of conditioned medium prepared by stimulation of hMSCs with IL-1β were incubated with different concentrations of a PGE2 blocking antibody (2B5; Cayman) for 30 minutes. After the 30-minute incubation, the immunosuppressive activity of the anti-PGE2 antibody-treated conditioned media was tested in IFN-γ ELISpot.
Results
Assessment of the immunomodulatory activity of hMSC conditioned medium
Conditioned medium from IL-1β- and IL-1β + TNF-α-stimulated hMSCs exhibited high (85 - 90% inhibition) immunosuppressive activity indicated by the decrease in the number of IFN-γ-positive spots. Conditioned media from unstimulated or TNF-α-stimulated hMSCs demonstrated limited (45-50% inhibition) immunosuppressive activity.
This same pattern of immunosuppressive activity was observed when serum-free medium with and without the inflammatory cytokines was used for the stimulation of hMSCs (Figure 1).
Figure 1.

Immunosuppresive activity of hMSC conditioned media. hMSCs were cultured for 24 hours in ITS+ -supplemented RPMI with IL-1β (5 pg/ml), TNF-α (25 ng/ml), or both IL-1β and TNF-α; unstimulated cultures were maintained in ITS+-supplemented RPMI. Control samples were prepared incubating the media with and without the cytokines in the absence of hMSCs. The immunosuppressive activity of the conditioned media was assessed in elispots.
Cytokine measurements in conditioned media
Analyses of the cytokine content of control conditioned media (unstimulated hMSCs) and conditioned media generated by activation of hMSCs with IL-1β, TNF-α or both IL-1β and TNF-α in serum-free medium revealed that the concentration of PGE2 in the conditioned media correlated (R2 = 0.74) with their immunosuppressive activity in IFN-γ ELISpot (Figure 2).
Figure 2.
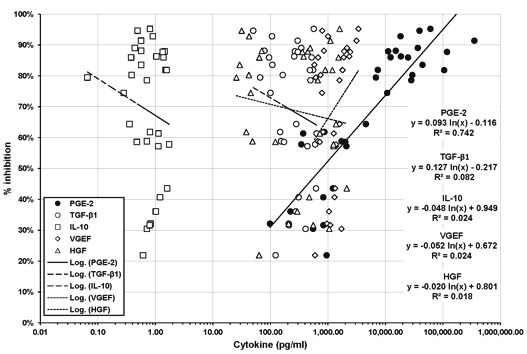
Correlation of cytokine concentration and immunsuppressive activity in hMSC conditioned media. The concentration of PGE2, TGF-β1, IL-10, VEGF and HGF in serum-free hMSC conditioned media was measured using Multiarray® kits. The concentration of the different cytokines was correlated with the immunosuppressive activity of the media. PGE2 was the only factor with a significant correlation between its concentration and the immunosuppresive activity of the media.
None of the other cytokines measured (HGF, TGF-β1, VEGF, IL-10) exhibited a similar level of correlation with the immunosuppressive activity of the conditioned media (Figure 2).
Stimulation of hMSCs with IL-1β alone resulted in an average 60-fold increase of PGE2 secretion compared to non-stimulated conditions; stimulation with TNF-α alone resulted in a 4-fold increase of PGE2 secretion compared to non-stimulated conditions; stimulation with IL-1β in combination with TNF-α resulted in 130-, 30- and 3-fold increase of PGE2 secretion compared to non-stimulated conditions, TNF-α stimulation and IL-1β stimulation respectively.
Immunosuppressive activity of exogenous PGE2
Analysis of the immunosuppressive activity of exogenous PGE2 added to either complete RPMI or ITS-supplemented RPMI in IFN-γ ELISpot demonstrated that PGE2 inhibits IFN-γ secretion by PHA-stimulated PBMCs in a dose-dependent manner (Figure 3). In three separate experiments performed with three independent PBMC preparations, the immunosuppressive activity of PGE2 reached maximum levels at concentrations ranging between 80 and 120 ng/ml.
Figure 3.
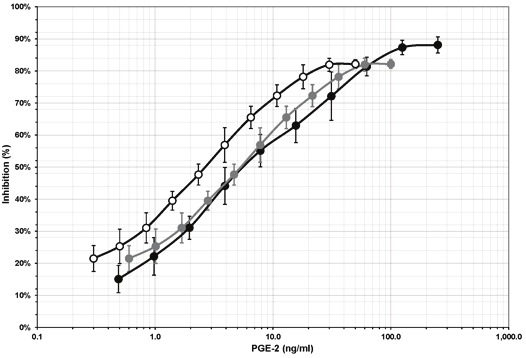
Immunosuppressive activity of exogenous PGE2. PGE2 was added to the medium in elispot assays. PGE2 was immunosuppressive in a dose-dependent manner. Data from three independent experiments.
Blocking PGE2 activity with antibodies
In three separate experiments performed with three different hMSC conditioned media and three independent PBMC preparations, incubation of serum-containing IL-1β-stimulated hMSC-conditioned media with a PGE2 blocking antibody blocked the immunosuppressive activity of the media in a dose-dependent manner (Figure 4).
Figure 4.
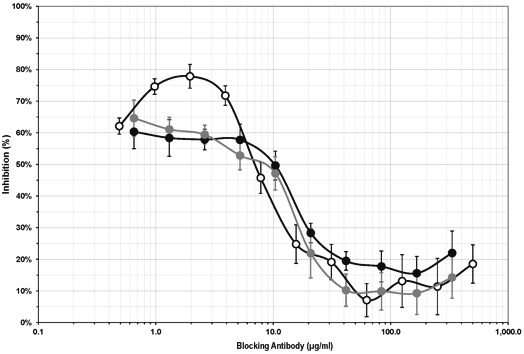
Inhibition of the immunosuppressive activity of hMSC conditioned media by a neutralizing anti-PGE2 antibody. hMSC conditioned media were pre-incubated with an anti-PGE2 antibody prior to assessing their immunosuppressive activity in elispot assays. The anti-PGE2 antibody blocked the immunosuppressive activity of the conditioned media in a dose-dependent manner. Data from three independent experiments.
Discussion
MSCs are non-hematopoietic cells derived from adult bone marrow that can differentiate into several mesenchymal tissues if specific in vitro and in vivo conditions are used [1]. MSCs are not immuno-stimulatory in vitro [12]. They do not induce lymphocyte proliferation when co-cultured with allogeneic lymphocytes and they are not targets for cytotoxic lymphocytes or NK-cells [13]. MSCs may also be tolerated when transplanted across major histocompatibility complex barriers in humans. In fact, in vitro findings indicate that MSCs are immunosuppressive [2]. Rodent, baboon or human MSCs suppress lymphocyte proliferation in mixed lymphocyte cultures (MLC) or by mitogens. They also inhibit the formation of cytotoxic T-cells and NK-cells. In a baboon model, MSCs delayed rejection of skin allografts [14].
Published reports indicate that the immunomodulatory potential of hMSCs exhibits variability among individuals and also as a function of culture conditions and time in culture [15]. It has also demonstrated that hMSCs can be effectively activated to secrete immunomodulatory factors by exposure to specific cytokines which allows more standardized conditions for the testing of the level of activity of a given cell preparation [15]. In these experiments we have identified serum-free conditions in which the stimulation of hMSCs to secrete immunomodulatory products is possible. This technical development greatly simplifies the analysis of the immunomodulatory factors secreted by the hMSCs upon activation by eliminating the noisy background of FBS supplementation.
The immunosuppressive activity of hMSCs conditioned media was tested in enzyme-linked immunosorbent spot assays (ELISpot) [16]. The ELISpot assay allows visualization of the secretory product of individual responding cells, each spot that develops in the assay represents a single reactive cell. Thus, the assay provides both qualitative (type of immune protein) and quantitative (number of responding cells) information. ELISpot assays are highly sensitive because the product is rapidly captured around the secreting cell before it is diluted in the supernatant, captured by receptors of adjacent cells, or degraded. The assay has gained a recent increase in popularity, especially as a surrogate measure for cytotoxic T-cell responses, in large part because it is both reliable and highly sensitive [17]. We have favored this assay over other assessments of T-cell activity because, as stated above, it is sensitive, reliable, simple, reproducible, and does not require the use of radioisotopes like traditional proliferation assays. Furthermore, we used a lectin (PHA) to stimulate the effector cells because of its polyclonal activating effect.
The data presented here indicate that PGE2 plays a critical role in the immunosuppressive activity of hMSCs. The role of PGE2 in regulation of T-cell function has been documented [18] and it has been suggested that it is one of the most important cytokines in the immunosuppressive activity of MSCs [19,20].
The results presented here should not be interpreted to mean that PGE2 is the only or even the principal mechanism by which hMSCs exert their immunomodulatory activity in vivo. In these experiments, we have focused on the active components present in medium conditioned by hMSCs exposed to pro-inflammatory molecules. Clearly, there are other molecules that have been postulated to play a significant role in the immunomodulatory activity of hMSCs which cannot be properly tested in this format [21]. Nitric Oxide (NO), for example, is one of the molecules postulated to be responsible for this activity [20-22]. However, NO is rapidly converted to nitrates or nitrites in solution and it is not likely that it would be an active molecule in the conditioned media tested in these experiments; its activity would likely only be effective when hMSCs and immune cells are in close proximity. Similarly, the potential role of indoleamine 2,3-digoxigenase (IDO) in the immunosuppressive activity of hMSCs would be limited to co-culture experiments because IDO is a cell surface enzyme that would not be present in cell-free conditioned media [22,23].
As stated above, the objective of these experiments was not to elucidate the molecular mechanism of hMSC immune modulation but rather to identify measureable analytes whose presence and concentration would correlate with immunosuppressive activity. Undoubtedly, PGE2 should be carefully explored as one of the molecules involved in this activity. These findings, however, need to be confirmed in vitro using different assays and, perhaps, different modalities of T-cell activation because there are reports indicating differences in the mechanism of action of hMSC modulation depending upon the type of activation [24] in vivo. Furthermore, these data will require in vivo validation. Cell preparations with different levels of activity as detected by these assays should be compared in vivo to determine the level of correlation of these in vitro data with their efficacy in preclinical models of immune disorders.
Acknowledgements
The author thanks Drs. Jeffery J Auletta and Wouter Van’t Hof for scientific discussions of these and other related experiments. This work was supported by a Large Pilot Grant of the Case Western Reserve University Clinical and Translational Science Collaborative Pilot Program. CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR) 2009-2010. This research was supported by the Hematopoietic Stem Cell Core Facility and the Translational Research Core Facility of the Case Comprehensive Cancer Center (P30 CA43703).
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 3.Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Auletta JJ, Deans RJ, Bartholomew AM. Emerging roles for multipotent, bone marrow-derived stromal cells in host defense. Blood. 2012;119:1801–1809. doi: 10.1182/blood-2011-10-384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auletta JJ, Cooke KR, Solchaga LA, Deans RJ, van't Hof W. Regenerative stromal cell therapy in allogeneic hematopoietic stem cell transplantation: current impact and future directions. Biol Blood Marrow Transplant. 2010;16:891–906. doi: 10.1016/j.bbmt.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 7.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. European Journal of Immunology. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 9.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 10.Lennon DP, Haynesworth SE, Bruder SP, Jaiswal N, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: Identification of serum for optimal selection and proliferation. In Vitro Cellular and Developmental Biology. 1996;32:602–611. [Google Scholar]
- 11.Lennon DP, Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1604–1605. doi: 10.1016/j.exphem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 13.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 14.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 15.Auletta JJ, Zale EA, Welter JF, Solchaga LA. Fibroblast Growth Factor-2 Enhances Expansion of Human Bone Marrow-Derived Mesenchymal Stromal Cells without Diminishing Their Immunosuppressive Potential. Stem Cells Int. 2011;2011:235176. doi: 10.4061/2011/235176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzymelinked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Caspell R, Karulin AY, Ahmad M, Haicheur N, Abdelsalam A, Johannesen K, Vignard V, Dudzik P, Georgakopoulou K, Mihaylova A, Silina K, Aptsiauri N, Adams V, Lehmann PV, McArdle S. ELISPOT assays provide reproducible results among different laboratories for T-cell immune monitoring-even in hands of ELISPOT-inexperienced investigators. J Immunotoxicol. 2009;6:227–234. doi: 10.3109/15476910903317546. [DOI] [PubMed] [Google Scholar]
- 18.Bao YS, Zhang P, Xie RJ, Wang M, Wang ZY, Zhou Z, Zhai WJ, Feng SZ, Han MZ. The regulation of CD4+ T cell immune responses toward Th2 cell development by prostaglandin E2. Int Immunopharmacol. 2011;11:1599–1605. doi: 10.1016/j.intimp.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Yanez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316:3109–3123. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87:S45–49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- 21.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Delarosa O, Lombardo E, Beraza A, Mancheno P, Ramirez C, Menta R, Rico L, Camarillo E, Garcia L, Abad JL, Trigueros C, Delgado M, Buscher D. Requirement of IFN-γ mediated Indoleamine 2,3 dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 23.Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681–693. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 24.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]


