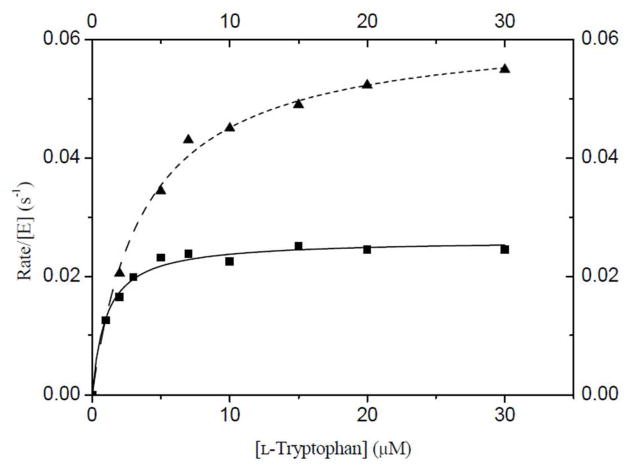
Figure 5.
Enzyme kinetic plots of initial velocity/[E] vs. substrate concentration demonstrating a solvent kinetic isotope effect. Tryptophan is the variable substrate and saturating DMAPP (20 μM) was employed. Triangles represent data obtained in H2O and squares represent data obtained in > 95% D2O. Data was fit to the Michaelis-Menten equation (dashed line = H2O and solid line = D2O).