Abstract
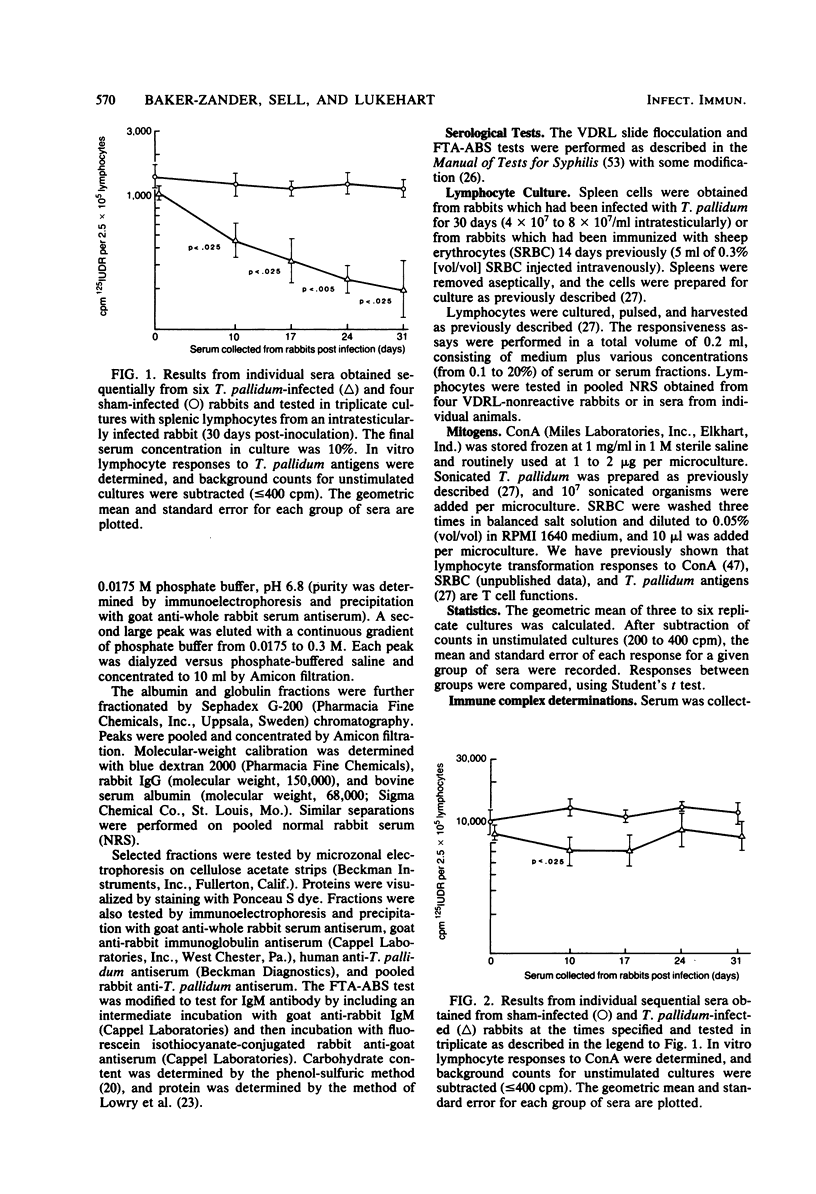
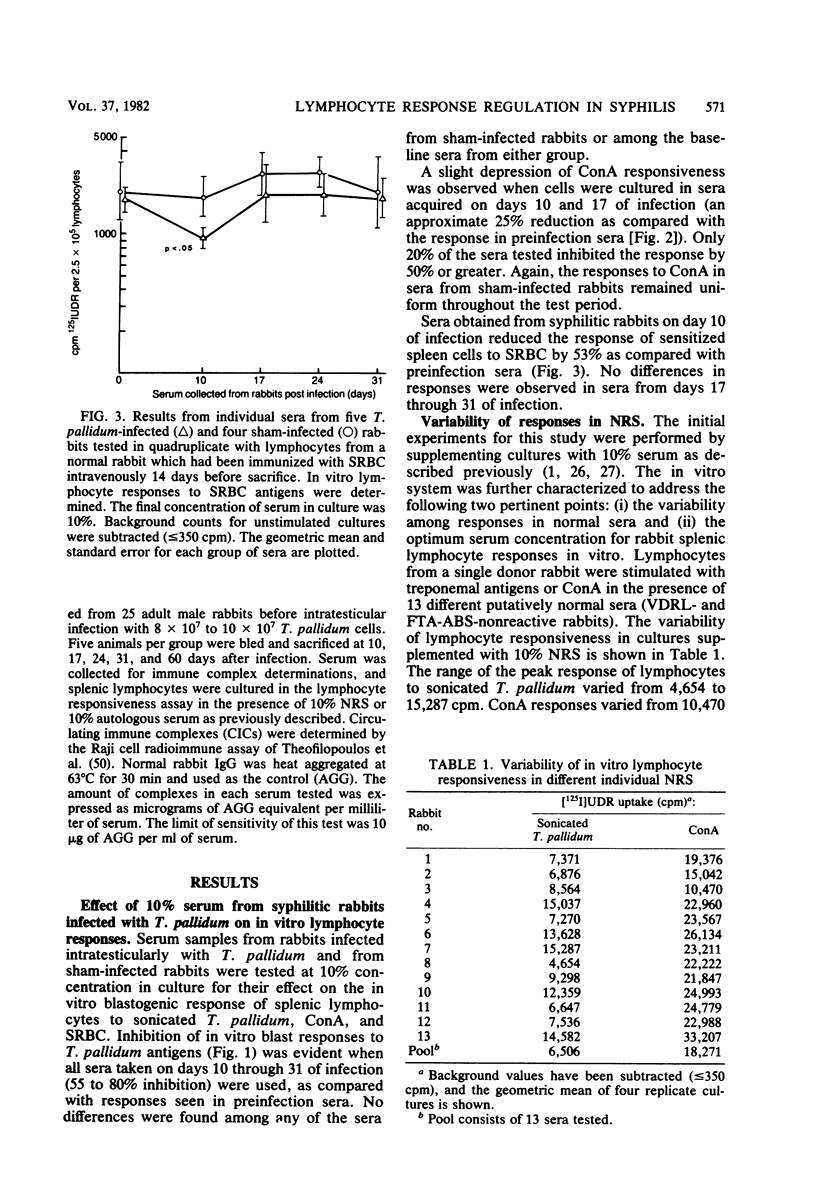
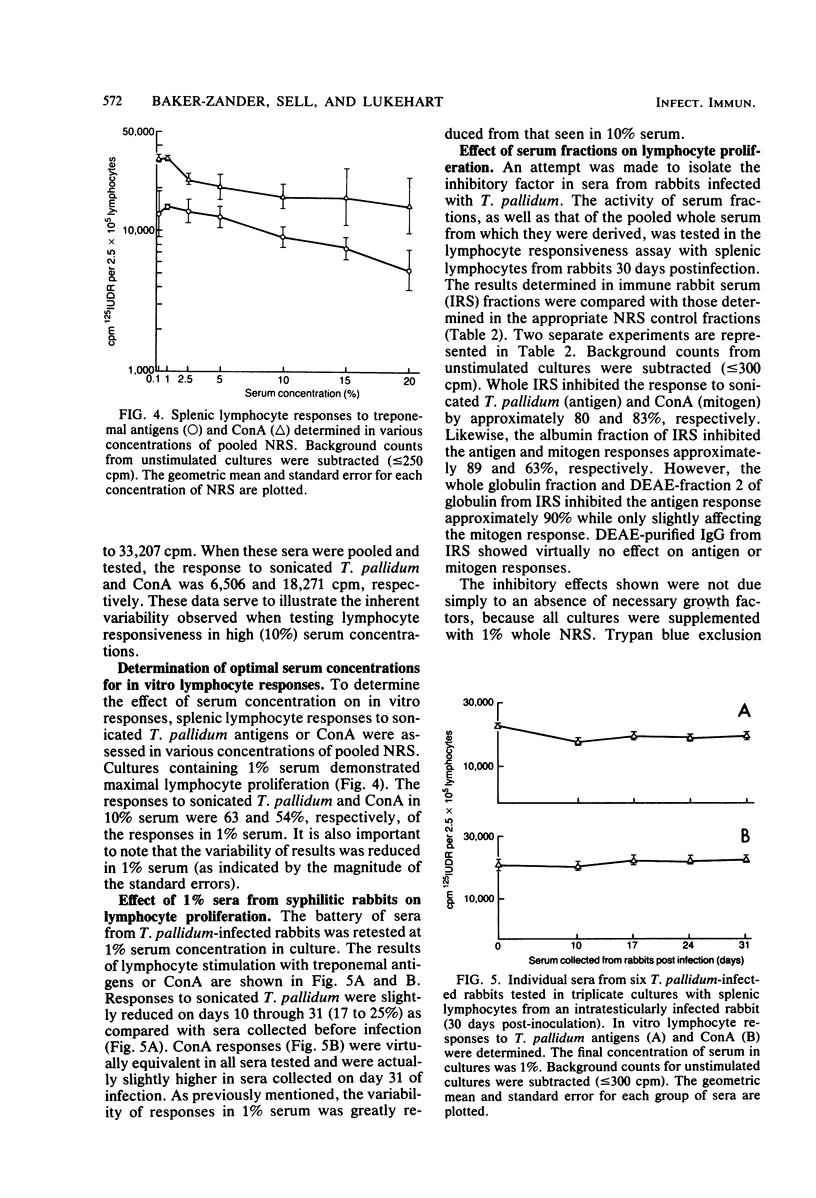
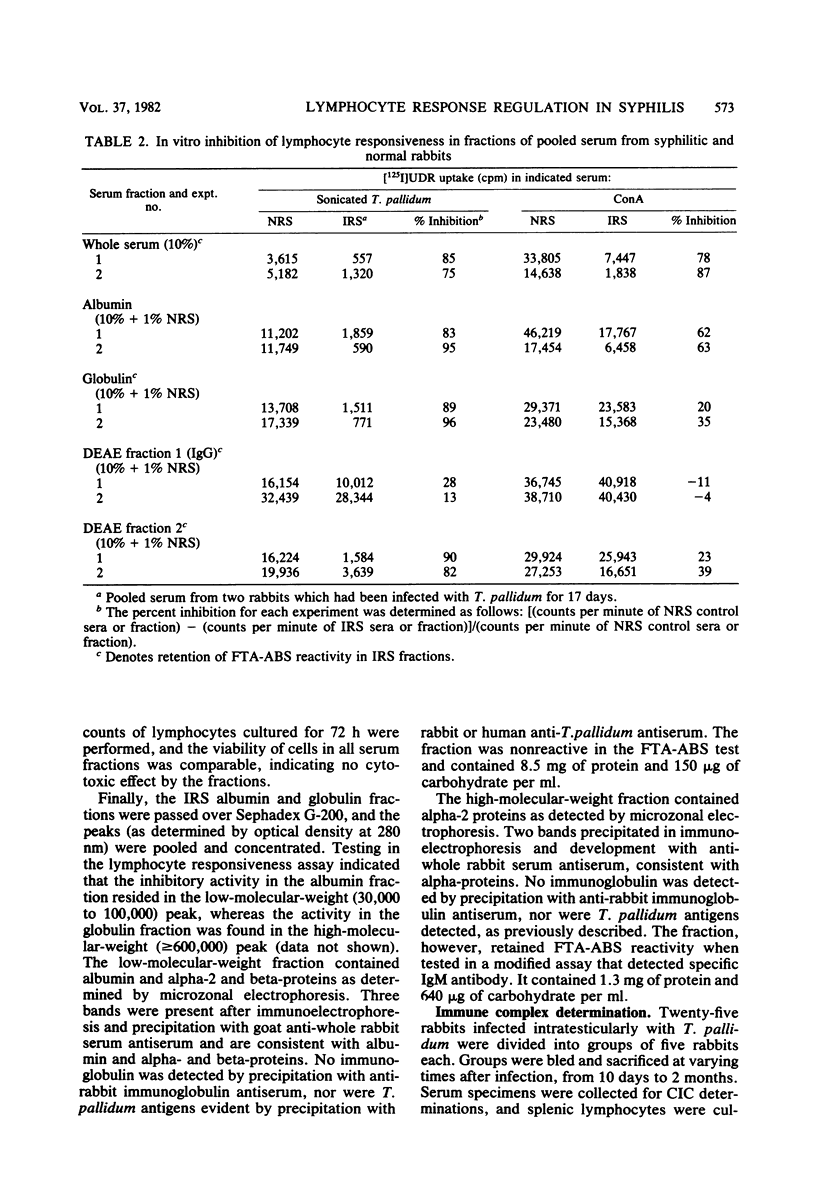
Sera from rabbits with early experimental syphilis were tested for their effect on in vitro lymphocyte transformation responses to related specific antigens (sonicated T. pallidum), unrelated specific antigens (sheep erythrocytes), and the T cell mitogen, concanavalin A. Results were compared with responses in preinfection sera and in sera from sham-infected rabbits. Titration experiments in which normal serum was used indicated that optimal lymphocyte responsiveness is obtained with a final serum concentration of 1%. Under these conditions, no differences in concanavalin A stimulation were observed in cultures with syphilitic sera. Responses to sonicated T. pallidum were inhibited, but only by 17 to 25% when compared with the response in preinfection sera. In cultures containing 10% serum, inhibition of lymphocyte proliferation to sonicated T. pallidum antigens was evident with sera from all syphilitic animals from day 10 (55% inhibition) through day 31 (80% inhibition) of infection. Responses to concanavalin A and sheep erythrocytes were significantly inhibited by day 10 sera; only 20% of the sera tested demonstrated substantial nonspecific inhibitory capacity. No differences were evident among sera from any of the sham-infected animals or among the preinfection sera from either group. Pooled serum with high inhibitory activity was fractionated by ammonium sulfate precipitation, DEAE ion exchange chromatography, and Sephadex G-200 gel filtration. Two separate inhibitors were identified: (i) a low-molecular-weight, ammonium sulfate-soluble, nonspecific inhibitory fraction containing albumin and alpha-globulins with the capacity to inhibit both antigen and mitogen responses and (ii) a high-molecular-weight, ammonium sulfate-precipitable, inhibitory fraction containing alpha-globulin and FTA-ABS-reactive immunoglobulin M which affected only the antigen-specific response to sonicated T. pallidum. Immunodiffusion failed to detect immunoglobulin or T. pallidum antigens in either fraction. DEAE-purified immunoglobulin G from immune serum was not inhibitory.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Zachar Z., Hayes N. S. Concanavalin A-mediated affinity film for Treponema pallidum. Infect Immun. 1980 Jan;27(1):260–263. doi: 10.1128/iai.27.1.260-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Reappraisal of lymphocyte responsiveness to concanavalin A during experimental syphilis: evidence that glycosaminoglycans in the sera and tissues interfere ith active binding sites on the lectin and not with the lymphocytes. Infect Immun. 1982 Feb;35(2):552–559. doi: 10.1128/iai.35.2.552-559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Tung K. S., Musher D. M. Detection of circulating immune complexes in the sera of rabbits with experimental syphilis: possible role in immunoregulation. Infect Immun. 1980 Aug;29(2):575–582. doi: 10.1128/iai.29.2.575-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C., Fitzgerald T. J. Suppression of lymphocyte response to concanavalin A by mucopolysaccharide material from Treponema pallidum-infected rabbits. Infect Immun. 1979 Oct;26(1):64–69. doi: 10.1128/iai.26.1.64-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. S. The effects of human serum fractions on phytohemagglutinin- and concanavalin A-stimulated human lymphocyte cultures. Cell Immunol. 1972 Dec;5(4):544–554. doi: 10.1016/0008-8749(72)90104-9. [DOI] [PubMed] [Google Scholar]
- Cooperband S. R., Badger A. M., Davis R. C., Schmid K., Mannick J. A. The effect of immunoregulatory globulin (IRA) upon lymphocytes in vitro. J Immunol. 1972 Jul;109(1):154–163. [PubMed] [Google Scholar]
- Cooperband S. R., Bondevik H., Schmid K., Mannick J. A. Transformation of human lymphocytes: inhibition by homologous alpha globulin. Science. 1968 Mar 15;159(3820):1243–1244. doi: 10.1126/science.159.3820.1243. [DOI] [PubMed] [Google Scholar]
- Cooperband S. R., Davis R. C., Schmid K., Mannick J. A. Competitive blockade of lymphocyte stimulation by a serum immuno-regulatory alpha globulin (IRA). Transplant Proc. 1969 Mar;1(1):516–523. [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Regulatory serum lipoproteins: regulation of lymphocyte stimulation by a species of low density lipoprotein. J Immunol. 1976 May;116(5):1452–1458. [PubMed] [Google Scholar]
- Engel S., Diezel W. Persistent serum immune complexes in syphilis. Br J Vener Dis. 1980 Aug;56(4):221–222. doi: 10.1136/sti.56.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennestad K. L., Bruun L., Wedø E. Pleural effusion disease agent as passenger of Treponema pallidum suspensions from rabbits. Survey of laboratories. Br J Vener Dis. 1980 Aug;56(4):198–203. doi: 10.1136/sti.56.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field E. J., Caspary E. A. Lymphocyte response depressive factor in multiple sclerosis. Br Med J. 1971 Nov 27;4(5786):529–532. doi: 10.1136/bmj.4.5786.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann P. S., Turk J. L. The role of cell-mediated immune mechanisms in syphilis in Ethiopia. Clin Exp Immunol. 1978 Jan;31(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- From E., Thestrup-Pedersen K., Thulin H. Reactivity of lymphocytes from patients with syphilis towards T. pallidum antigen in the leucocyte migration and lymphocyte transformation tests. Br J Vener Dis. 1976 Aug;52(4):224–229. doi: 10.1136/sti.52.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCHHORN K., SCHREIBMAN R. R., BACH F. H., SILTZBACH L. E. IN-VITRO STUDIES OF LYMPHOCYTES FROM PATIENTS WITH SARCOIDOSIS AND LYMPHOPROLIFERATIVE DISEASES. Lancet. 1964 Oct 17;2(7364):842–843. doi: 10.1016/s0140-6736(64)90691-9. [DOI] [PubMed] [Google Scholar]
- Hsu C. C., Leevy C. M. Inhibition of PHA-stimulated lymphocyte transformation by plasma from patients with advanced alcoholic cirrhosis. Clin Exp Immunol. 1971 May;8(5):749–760. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letter: Anergy in patients with syphilis. N Engl J Med. 1975 Jul 10;293(2):99–100. doi: 10.1056/NEJM197507102930217. [DOI] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A. Activation of macrophages by products of lymphocytes from normal and syphilitic rabbits. Infect Immun. 1982 Jul;37(1):64–69. doi: 10.1128/iai.37.1.64-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Effect of cortisone administration on host-parasite relationships in early experimental syphilis. J Immunol. 1981 Oct;127(4):1361–1368. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- Maret S. M., Baseman J. B., Folds J. D. Cell-mediated immunity in Treponema pallidum infected rabbits: in vitro response of splenic and lymph node lymphocytes to mitogens and specific antigens. Clin Exp Immunol. 1980 Jan;39(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- Milton J. D., Mowbray J. F. Reversible loss of surface receptors on lymphocytes. Immunology. 1972 Oct;23(4):599–608. [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M. Evidence that specific oligosaccharides block early events necessary for the expression of antigen-specific proliferation by human lymphocytes. J Immunol. 1980 Sep;125(3):1306–1311. [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. In vitro lymphocyte response to Treponema refringens im human syphilis. Infect Immun. 1974 Apr;9(4):654–657. doi: 10.1128/iai.9.4.654-657.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. S. Mouse serum factor depressing lymphocyte transformation. Experientia. 1972 Oct 15;28(10):1227–1228. doi: 10.1007/BF01946187. [DOI] [PubMed] [Google Scholar]
- Nelson D. S., Nelson M., Thurston J. M., Waters M. F., Pearson J. M. Phytohaemagglutinin-induced lymphocyte transformation in leprosy. Clin Exp Immunol. 1971 Jul;9(1):33–43. [PMC free article] [PubMed] [Google Scholar]
- Nelson D. S., Shneider C. N. Effect of normal mouse serum on mouse lymphocyte transformation in vitro. Eur J Immunol. 1974 Feb;4(2):79–86. doi: 10.1002/eji.1830040205. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Modulation of in vitro lymphocyte transformation by antibodies: enhancement by antigen-antibody complexes and inhibition by antibody excess. Cell Immunol. 1972 Mar;3(3):341–360. doi: 10.1016/0008-8749(72)90243-2. [DOI] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Electron microscopy of phagocytosis in syphilis and yaws. Br J Vener Dis. 1972 Aug;48(4):227–248. doi: 10.1136/sti.48.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Selective in vitro response of thymus-derived lymphocytes from Treponema pallidum-infected rabbits. Infect Immun. 1977 Dec;18(3):603–611. doi: 10.1128/iai.18.3.603-611.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistole T. G. Interaction of bacteria and fungi with lectins and lectin-like substances. Annu Rev Microbiol. 1981;35:85–112. doi: 10.1146/annurev.mi.35.100181.000505. [DOI] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S. A., Lloyd R. M. T-cell hyperplasia of lymphoid tissues of rabbits infected with Treponema pallidum: evidence for a vigorous immune response. Sex Transm Dis. 1980 Apr-Jun;7(2):74–84. doi: 10.1097/00007435-198004000-00009. [DOI] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S., Powell H. C. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982 Apr;46(4):355–364. [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Jr, Redelman D., Sell S. In vitro studies of the rabbit immune system. IV. Differential mitogen responses of isolated T and B cells. Cell Immunol. 1976 Jun 1;24(1):34–44. doi: 10.1016/0008-8749(76)90129-5. [DOI] [PubMed] [Google Scholar]
- Sølling J., Sølling K., Jakobsen K. U., From E. Circulating immune complexes in syphilis. Acta Derm Venereol. 1978;58(3):263–267. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Wilson C. B., Dixon F. J. The Raji cell radioimmune assay for detecting immune complexes in human sera. J Clin Invest. 1976 Jan;57(1):169–182. doi: 10.1172/JCI108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. J., Mangi R. J., Lee R., Dwyer J. M. Immunoregulatory properties of serum from patients with different stages of syphilis. Br J Vener Dis. 1980 Aug;56(4):210–217. doi: 10.1136/sti.56.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Modulation of normal lymphocyte stimulation by serum from Treponema pallidum-infected rabbits. Cell Immunol. 1980 Jul 15;53(1):29–38. doi: 10.1016/0008-8749(80)90423-2. [DOI] [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Serum of rabbits infected with Treponema pallidum (Nichols) inhibits in vitro transformation of normal rabbit lymphocytes. Cell Immunol. 1979 Feb;42(2):363–372. doi: 10.1016/0008-8749(79)90201-6. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Yachnin S. Inhibition of phytohemagglutinin-induced lymphocyte transformation by globulins; lack of correlation with phytohemagglutinin precipitation by serum proteins. J Immunol. 1972 Mar;108(3):845–847. [PubMed] [Google Scholar]
- de Jong N. J., Koehorst J. A., van der Sluis J. J., Boer A. M. Autolymphocytotoxins in syphilis. Br J Vener Dis. 1980 Oct;56(5):297–301. doi: 10.1136/sti.56.5.297. [DOI] [PMC free article] [PubMed] [Google Scholar]


