Table II.
Summary BE Statistics for Two HV Generic Drugs, Analyzed Using RSABE or Unscaled ABE, Depending upon the Value of s WR
| Summary of statistical analysis-scaled data: least-square geometric means, point estimates and 90% confidence intervals | ||||||||
| Parameter | T/R ratio | Lower 90% CL | Upper 90% CL |
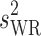
|
s WR | Criteria bound | Method used | Outcome |
| Drug A: fed bioequivalence study, N = 43 | ||||||||
| AUC0 − t | 1.17 | 93.91 | 132.91 | 0.5723 | 0.7565 | −0.2892 | Scaled/PE | Pass |
| AUC∞ | 1.11 | 94.64 | 124.91 | 0.3452 | 0.5875 | −0.1767 | Scaled/PE | Pass |
| C max | 1.19 | 100.47 | 133.26 | 0.3768 | 0.6138 | −0.1684 | Scaled/PE | Pass |
| Drug B: fasting bioequivalence study, N = 36 | ||||||||
| AUC0 − t | 0.99 | 89.82 | 109.53 | 0.07425 | 0.2725 | −0.03914 | Unscaled | Pass |
| AUC∞ | 1.02 | 92.57 | 111.77 | 0.06561 | 0.2561 | −0.03126 | Unscaled | Pass |
| C max | 0.98 | 85.70 | 110.54 | 0.1069 | 0.3270 | −0.05294 | Scaled | Pass |
