Fig. 3.
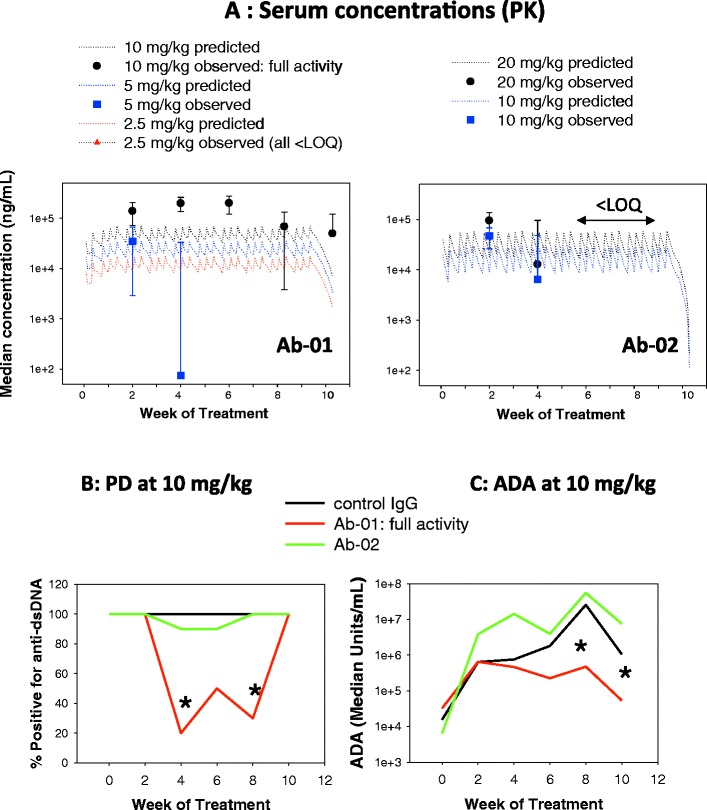
Example of interdependencies in PK, PD, and ADA data in preclinical studies of a biotherapeutic. Twelve-week-old MRL-Fas lpr mice were administered anti-IL-21R antibody Ab-01 (10, 5, or 2.5 mg/kg), anti-IL-21R antibody Ab-02 (20 or 10 mg/kg), or an isotype control antibody (10 mg/kg) by intraperitoneal (IP) injection three times per week over 10 weeks and sacrificed ~3 days after the last dose. Serum was collected prior to dosing (week 0) and then at 2, 4, 6, 8, and 10 weeks post-dose. Sera were analyzed for test article concentration (panel A), anti-dsDNA biomarker (panel B), and ADAs (panel C) by immunoassays. Panel A shows observed median ± standard deviations (symbols with error bars) and predicted concentrations (dotted lines; based on simulations using serum concentration data of Ab-01 and Ab-02 observed after a single IP dose to MRL-Fas lpr mice). Asterisks indicate a significant difference in anti-dsDNA biomarker (B) and ADA (C), as compared to the isotype control group. IgG deposits in the kidney sections were assessed by immunohistochemistry at sacrifice (not shown), and only the 10-mg/kg Ab-01 dose group showed significant reduction in IgG deposits (referred to as “full activity”). These data were compiled based on the results reported by Vugmeyster et al. (7) PK pharmacokinetics, PD pharmacodynamics, ADA anti-drug antibodies, LOQ limit of quantification, anti-dsDNA anti-double-stranded DNA antibodies
