Abstract
Regulation by abscisic acid (ABA) and Ca2+ of broad bean (Vicia faba) abaxial and adaxial guard cell movements and inward K+ currents were compared. One millimolar Ca2+ in the bathing medium inhibited abaxial stomatal opening by 60% but only inhibited adaxial stomatal opening by 15%. The addition of 1 μm ABA in the bathing medium resulted in 80% inhibition of abaxial but only 45% inhibition of adaxial stomatal opening. Similarly, ABA and Ca2+ each stimulated greater abaxial stomatal closure than adaxial stomatal closure. Whole-cell patch-clamp results showed that the inward K+ currents of abaxial guard cells were inhibited by 60% (−180 mV) in the presence of 1.5 μm Ca2+ in the cytoplasm, whereas the inward K+ currents of adaxial guard cells were not affected at all by the same treatment. Although 1 μm ABA in the cytoplasm inhibited the inward K+ currents to a similar extent for both abaxial and adaxial guard cells, the former were more sensitive to ABA applied externally. These results suggest that the abaxial stomata are more sensitive to Ca2+ and ABA than adaxial stomata in regard to stomatal opening and closing processes and that the regulation of the inward K+ currents by ABA may not proceed via a Ca2+-signaling pathway in adaxial guard cells. Therefore, there may be different pathways for ABA- and Ca2+-mediated signal transduction in abaxial and adaxial guard cells.
Regulation of stomatal apertures controls gas exchange between plant leaves and the environment, thereby influencing plant metabolism and overall plant growth and development. It is well known that stomatal apertures are regulated by a number of environmental and physiological factors, including humidity, plant water status, CO2 concentration, light intensity and quality, cytoplasmic Ca2+ concentration, and ABA concentration (Zeiger et al., 1987; Kearns and Assmann, 1993). Therefore, the study of physiological mechanisms of stomatal movements and their regulation has great importance for understanding signal transduction mechanisms in plant cells (Assmann, 1993; Chasan, 1995; Willmer and Fricker, 1996b). Stomatal movement (changes in stomatal aperture) depends on the swelling and shrinking of the guard cells around the stomatal pores, which is caused by changes in the turgor of the cells. The changes in guard cell turgor are brought about by changes in K+ and anion fluxes across the plasma membranes, as well as by organic ion synthesis in the guard cell cytoplasm (Assmann, 1993; Blatt and Grabov, 1997).
Stomatal opening normally occurs when an increase in guard cell osmotica drives water influx, cell volume increase, and a separation of the guard cell pair (Zeiger et al., 1987). K+ uptake plays a significant role in this process and is promoted by a hyperpolarized membrane potential created by plasma membrane H+-ATPases that pump H+ out of the guard cell cytoplasm (Assmann et al., 1985; Shimazaki et al., 1986; Hedrich and Schroeder, 1989). Stomatal closure is not exactly the reverse of stomatal opening but is accompanied by K+ efflux through Kout channels in the guard cell plasma membrane and by inactivation of Kin channels (Schroeder and Fang, 1991; Kearns and Assmann, 1993; McAinsh et al., 1997).
Most herbaceous plants have stomata on both the abaxial and adaxial sides of their leaves. There are a number of differences between abaxial and adaxial stomata. First, stomatal density is usually higher on the abaxial surface than it is on the adaxial surface of leaves (Willmer and Fricker, 1996a). Second, morphological differences in the guard cells and stomatal pores between abaxial and adaxial stomata are also significant: abaxial guard cells are typically larger (Willmer and Fricker, 1996b) and stomatal pores are wider under conditions favoring opening. Third, gas exchange between a leaf or leaflet and the atmosphere occurs mainly via abaxial stomata, whereas adaxial stomata play a more minor role in gas-exchange processes (Lu, 1988). More importantly, sensitivities of stomatal movements in response to environmental stimuli differ significantly between the two cell types. This may reflect the fact that abaxial guard cells are usually more sensitive to environmental signals such as changes in light intensity or quality, soil water status, ambient humidity, and CO2 concentration (Lu, 1988; Lu et al., 1993; Goh et al., 1995).
Differences remain even when the two epidermes are excised from the leaves; the different sensitivities of abaxial and adaxial stomata to environmental signals can be observed in vitro (Travis and Mansfield, 1981; Pemadasa, 1982). This indicates that the differences in stomatal sensitivities to environmental stimuli cannot be attributed exclusively to different microenvironments in situ (De Silva et al., 1986). Travis and Mansfield (1981) demonstrated that the sensitivity of stomatal movement in isolated Commelina communis abaxial epidermes to changes in light intensity was much greater than that seen in adaxial epidermes. Abaxial stomata were also more sensitive than adaxial stomata in their response to changes in light quality (Pemadasa, 1982). Goh et al. (1995) provided evidence that maximal H+-pumping activities in broad bean (Vicia faba) abaxial and adaxial guard cells were similar, but the H+-pumping activity in adaxial guard cells required a much higher light intensity to reach the same level as abaxial guard cells. De Silva et al. (1986) suggested that cytoplasmic Ca2+ concentrations may be different in abaxial and adaxial guard cells, which might result in different sensitivities to environmental signals. All of these observations imply that there may be different signal transduction systems or pathways in abaxial and adaxial guard cells.
In the past decade a number of studies have focused on studying the mechanisms of signal transduction in isolated guard cell protoplasts. However, most of these studies used a mixed guard cell population obtained from both the abaxial and adaxial epidermes of plant leaves, as is provided by the “blender method” of guard cell protoplast isolation (Kruse et al., 1989). In our previous patch-clamp studies with guard cells, we noticed significant variation in the magnitude of the K+ current from cell to cell. It has been known for some time that the inward K+ currents of guard cells are inhibited when the cytoplasmic Ca2+ concentration increases (Schroeder and Hagiwara, 1989; Blatt et al., 1990; Fairley-Grenot and Assmann, 1992; Lemtri-Chlieh and MacRobbie, 1994). However, some guard cells from a single protoplast preparation do not respond to even millimolar levels of Ca2+ applied to the cytosol in whole-cell patch-clamp experiments (W.H. Wu and S.M. Assmann, unpublished data). It is essentially impossible to distinguish abaxial guard cells from adaxial guard cells under light microscopy; therefore, it is likely that both abaxial and adaxial guard cell protoplasts have been used for patch-clamp recordings (Luan et al., 1993; Kelly et al., 1995). It is reasonable to speculate that the variation observed in the electrophysiological experiments with guard cells may be attributed, at least in part, to variations in the cells used for the recordings, i.e. either abaxial or adaxial guard cell protoplasts.
To clarify whether there are different signal transduction mechanisms mediated by ABA and Ca2+ in abaxial versus adaxial guard cells, we applied patch-clamp techniques to study Kin channel regulation by ABA and Ca2+ in abaxial and adaxial guard cells. Effects of ABA, Ca2+, and water stress on abaxial and adaxial stomatal conductance (G) and stomatal apertures were also monitored to correlate single-cell and whole-leaf responses.
MATERIALS AND METHODS
Plant Growth Conditions
Plants of broad bean (Vicia faba L. cv Chong-Li) were grown from seeds that had been soaked in water for 4 d and then planted in potting mix (rich soil:vermiculite = 2:1, v/v) in growth chambers. The light intensity was 0.160 to 0.180 mmol m−2 s−1 for a 12-h daily light period, and day/night temperatures were 25°C ± 2°C and 20°C ± 2°C, respectively.
Stomatal Conductance and Stomatal Aperture Measurements
Stomatal conductances were measured from fully expanded young leaves oriented so that either the abaxial or adaxial surface was facing the measuring chamber of a steady-state porometer (model LI-1600, Li-Cor, Lincoln, NE). Measurements were made in the growth chamber between 10 and 11 am. The light intensity was 0.180 mmol m−2 s−1 and the temperature was 22°C. For experiments on the effect of water stress on abaxial and adaxial stomatal conductances, plants were water stressed by withholding water for 3 d. The presence of water stress was confirmed by the observation at both 10 am and 4 pm of a 30% decrease in stomatal aperture in epidermal peels taken from water-stressed plants relative to those taken from control plants. These experiments were repeated three times.
For epidermal peel experiments, abaxial or adaxial epidermal strips were peeled from fully expanded young leaves and incubated in medium containing 10 mm Mes-KOH, pH 6.0, and 50 mm KCl with or without Ca2+ or ABA. After a 2-h incubation at 22°C in the dark or in light with a fluence rate of 0.170 mmol m−2 s−1 (fluorescent bulbs, Philips, Eindhoven, The Netherlands), stomatal apertures were measured under a microscope. The percentage of ruptured epidermal cells resulting from the peeling process was determined by staining peeled epidermes with fluorescein diacetate dye and was checked under a fluorescent microscope with blue light.
Preparation of Guard Cell Protoplasts
Guard cell protoplasts were isolated from the epidermes of young expanded leaves from 3- to 4-week-old plants. The abaxial epidermes were completely peeled from each leaflet and cut into small pieces for the isolation of abaxial guard cells. The remaining leaflet pieces, devoid of abaxial epidermis, were processed in a blender (Waring) in a medium containing 10 mm Mes-KOH, pH 5.5, 5 mm CaCl2, 0.5 mm ascorbic acid, and 0.1% (w/v) PVP-40 twice for 30 s each to isolate adaxial epidermes. Abaxial and adaxial epidermes were then separately digested in enzyme solutions using a two-step method.
The epidermes were first digested in enzyme solution containing 0.7% (w/v) Cellulysin (Calbiochem), 0.1% (w/v) PVP-40, and 0.25% (w/v) BSA in basic medium (5 mm Mes, pH 5.5, 0.5 mm CaCl2, 0.5 mm MgCl2, 10 μm KH2PO4, 0.5 mm ascorbic acid, and 0.45 m sorbitol) at 25°C in a water-bath shaker with a shaking speed of 120 rpm. After a 30-min digestion, the epidermes were transferred into a second enzyme solution containing 1.5% (w/v) Cellulase RS (Yakult Honsha, Tokyo, Japan), 0.02% (w/v) Pectolyase Y-23 (Seishin Pharmaceutical, Tokyo, Japan), and 0.25% (w/v) BSA in basic medium. The second enzymatic digestion was conducted at 22°C for 60 to 80 min with the shaking speed at 60 to 70 rpm. The digested mixture was then filtered through a nylon net with a 22-μm pore size, washed with basic medium, and centrifuged at 150g for 5 min. The pellet was resuspended with basic medium and given a second centrifugation at 200g for 5 min. The pelleted guard cell protoplasts were resuspended with a small volume of basic medium and kept on ice for the patch-clamp experiments.
Patch-Clamping Procedure
In this study we used standard whole-cell recording techniques (Hamill et al., 1981). For a given experiment, the same leaves were used to isolate both abaxial and adaxial guard cell protoplasts, and experiments were conducted with both cell types at the same time of day. To confirm that the differential mechanical stresses experienced during the isolation of adaxial versus abaxial guard cell protoplasts did not account for the differences reported here between the two cell types, patch-clamp experiments were also performed on abaxial guard cell protoplasts from epidermes that had been hand-peeled and blended as for the adaxial epidermes. Results obtained from abaxial guard cell protoplasts prepared in this manner were identical (data not shown) to those reported here.
Guard cell protoplasts were placed in a bath solution (10 mm Hepes, pH 6.0, 10 mm potassium glutamate, 1 mm CaCl2, and 4 mm MgCl2; osmolality at 450 mmol kg−1 adjusted with sorbitol) with a final K+ concentration of 10.4 mm. Glass pipettes pulled from glass capillaries (Kimax-51, Kimble, Vineland, NJ) and heat-polished before use had resistances of approximately 20 MΩ when filled with the pipette solution (10 mm Hepes, pH 7.2, 98 mm potassium glutamate, 2 mm KCl, 2 mm EGTA, and 2 mm Mg-ATP; osmolality at 500 mmol kg−1 adjusted with sorbitol). The final K+ concentration for this pipette solution was 107 mm. For the internal high-Ca2+ treatment, 1.82 mm CaCl2 was added in the pipette solution, and the calculated free Ca2+ concentration was 1.5 μm (the calculation was conducted using Max Chelator, version 5.60, software developed by Dr. Chris Patton at Stanford University). Whole-cell clamping was performed at room temperature (20°C ± 2°C) in the dark. Seal resistance was between 1 and 3 GΩ in all experiments. Cell capacitance was measured for each cell using the capacity-compensation device of the amplifier (Bookman et al., 1991) and was between 4.0 and 7.2 pF. Data were acquired 10 min after the formation of the whole-cell configuration.
Whole-cell currents were measured using an amplifier (Axopatch-200A, Axon Instruments, Foster City, CA) connected to a microcomputer via an interface (TL-1 DMA Interface, Axon Instruments). pCLAMP (version 6.0.2, Axon Instruments) software was used to acquire and analyze the whole-cell currents. After the whole-cell configuration was obtained, membrane potential was clamped to −58 mV (holding potential). Voltage pulse protocols shown in Figure 1A were generated using pCLAMP software and applied to the clamped cell during data acquisition. Whole-cell currents were filtered at 1 kHz by a four-pole Bessel filter before storage (1 ms per sample) on a computer disk.
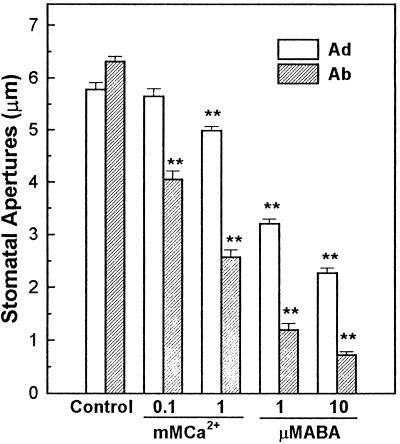
Figure 1.
Inhibition of stomatal opening of adaxial (Ad) and abaxial (Ab) epidermes by ABA and Ca2+. Broad bean leaves were removed from plants in the dark, and the epidermal tissues were peeled and kept in the dark in bathing medium for 1 h before experiments. Stomatal apertures were measured under a light microscope after the epidermes were illuminated for 2 h under 0.160 mmol m−2 s−1 white light. Either Ca2+ or ABA was added to the bathing medium at the concentrations indicated. The experiment was repeated three times. Data are expressed as means ± se (n = 60). The results for each Ca2+ or ABA treatment were compared with the control, and the statistical analysis (t test) was conducted at the P ≤ 0.01 (**) level.
Leak currents were subtracted before whole-cell current-voltage relations were generated. Leak currents for each cell were defined from the first one to three data points obtained after the membrane potential was stepped from the holding voltage to the test voltages. The mean values of time-activated whole-cell currents were determined as the average of samples obtained between 1.4 and 1.9 s (500 samples total) after imposition of the test voltage (i.e. when the current amplitude had reached a plateau). After leak currents were subtracted, the final whole-cell currents were expressed as currents per unit capacitance (pA pF−1) to account for variations in the cell surface area.
Chemicals
All chemicals were obtained from Sigma unless otherwise indicated.
RESULTS
Morphological Comparison of Abaxial and Adaxial Guard Cell Protoplasts
There were no significant morphological differences between the two types of protoplasts under light microscopy (photomicrographs not shown). The only morphological difference observed at the subcellular level using electron microscopy was within the chloroplasts, where granular thylakoids could be seen in abaxial but not in adaxial guard cells (photomicrographs not shown).
Effects of External ABA and Ca2+ on Stomatal Apertures in Abaxial and Adaxial Epidermes
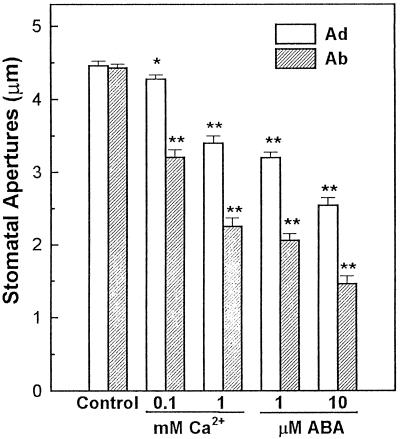
The inhibitory effects of ABA and Ca2+ on opening of stomata in abaxial and adaxial epidermal tissues were determined, and the results are shown in Figure 1. In the presence of 1 mm Ca2+ in the bathing medium, stomatal opening in the abaxial epidermes was inhibited by 60%, whereas stomatal opening for adaxial epidermes was only inhibited by 15%. Similarly, the addition of 1 μm ABA in the bathing medium resulted in 80% inhibition of stomatal opening in abaxial epidermes but caused only 45% inhibition of stomatal opening for adaxial epidermes. Figure 2 shows the effects of ABA and Ca2+ on the promotion of stomatal closure. In the presence of 1 mm Ca2+ or 1 μm ABA in the bath, stomatal apertures on the abaxial epidermis were 50% and 46% of the control, respectively, whereas stomatal apertures on the adaxial epidermis were 76% and 72% of the control, respectively. The results of the statistical analysis are shown in Figures 1 and 2. The results suggest that the abaxial stomata are more sensitive to Ca2+ and ABA in both stomatal opening and closing processes.
Figure 2.
Promotion of stomatal closing in adaxial (Ad) and abaxial (Ab) epidermes by ABA and Ca2+. Broad bean leaves were removed from plants in the light, and the epidermal tissues were peeled and kept in the light (0.160 mmol m−2 s−1) in bathing medium for 1 h before experiments. Stomatal apertures were measured under a light microscope after the epidermes were treated for 2 h in the dark. Either Ca2+ or ABA was added to the bathing medium at the concentrations indicated. The experiment was repeated three times. Data are expressed as means ± se (n = 60). The results for each Ca2+ or ABA treatment were compared with the control, and the statistical analysis (t test) was conducted at the P ≤ 0.05 (*) or the P ≤ 0.01 (**) level.
To determine whether the different percentages of ruptured epidermal cells would affect the stomatal apertures during the treatments, the peeled or blended abaxial and adaxial epidermes were stained with fluorescein diacetate dye and checked under a fluorescent microscope and blue light. The percentages of ruptured epidermal cells were 70% to 75% for the adaxial epidermis and 50% for the abaxial epidermis obtained by peeling and 90% to 95% for both adaxial and abaxial epidermes obtained by a brief blending. For Ca2+ and ABA treatments, stomatal apertures were not affected by the methods used for obtaining epidermis. Therefore, the percentages of ruptured epidermal cells may not significantly affect the stomatal apertures during the treatments.
Effects of Internal ABA and Ca2+ on Inward K+ Currents of Abaxial and Adaxial Guard Cell Protoplasts
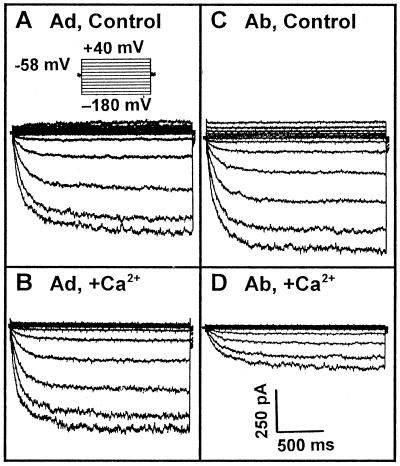
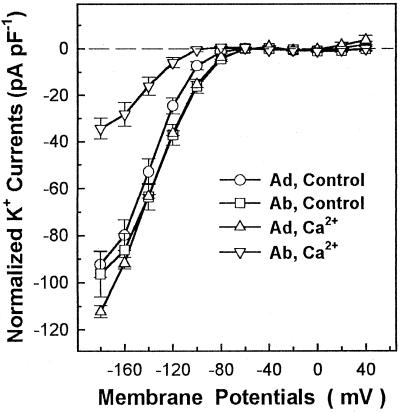
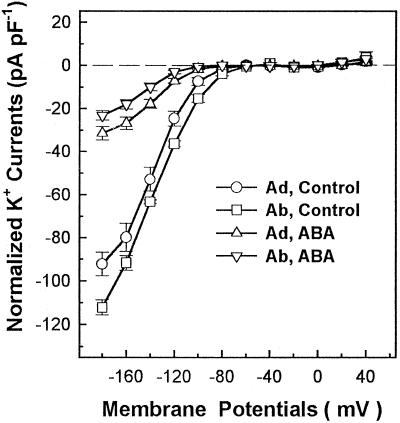
Figure 3 shows actual whole-cell recordings of guard cell inward K+ currents in the absence or presence of 1.5 μm Ca2+ (the calculated free Ca2+ concentration) in the recording pipettes. Different sensitivities of abaxial and adaxial guard cell inward K+ currents to cytoplasmic Ca2+ are clearly shown in Figure 4. The inward K+ currents of abaxial guard cells at −160 mV were inhibited by 67% in the presence of 1.5 μm Ca2+ in the pipette solution, and the differences of the currents at −160 mV between the absence and presence of 1.5 μm Ca2+ were significant by t test at P ≤ 0.01. However, the inward K+ currents of adaxial guard cells were not affected at all by the presence of 1.5 μm Ca2+ in the pipettes, and the differences of the currents at −160 mV in the absence and presence of 1.5 μm Ca2+ were not significant by t test at P ≤ 0.05. Figure 5 shows that the addition of 1 μm ABA to the cytosol via the patch pipette solution inhibited the inward K+ currents in both abaxial and adaxial guard cells to almost the same extent. The statistical analysis (t test) showed that the differences of the currents at −160 mV between the absence and presence of 1 μm ABA in the pipettes were significant at P ≤ 0.01 for both abaxial and adaxial guard cells.
Figure 3.
Whole-cell recordings of broad bean adaxial (Ad; A and B) and abaxial (Ab; C and D) guard cell protoplasts with (B and D) or without (A and C) 1.5 μm Ca2+ (the calculated free Ca2+ concentration) in the pipette solutions. Voltage protocols are shown as in A, and the current and time scale bars are shown in D for all recordings.
Figure 4.
Current/voltage relations of whole-cell inward K+ currents with adaxial (Ad) and abaxial (Ab) guard cell protoplasts with or without 1.5 μm Ca2+ (the calculated free Ca2+ concentration) in the pipette solutions. K+ current amplitudes are expressed as the current per unit cell capacitance to correct for variations resulting from different cell sizes. The number of replicates for each treatment were 9 (Ad, Control), 11 (Ad, Ca2+), and 6 (Ab, Control and Ab, Ca2+). Each data point is the mean ± se.
Figure 5.
Current/voltage relations of whole-cell inward K+ currents with adaxial and abaxial guard cell protoplasts with or without 1.0 μm ABA in the pipette solutions. K+ current amplitudes are expressed as the current per unit cell capacitance to correct for variations resulting from different cell sizes. The number of replicates for each treatment were 9 (Ad, Control), 14 (Ad, ABA), 6 (Ab, Control), and 10 (Ab, ABA). Each data point is the mean ± se.
The effects of internal Ca2+ and ABA on the V1/2 and the Gmax (asymptotic value) of the Kin channels at the saturated voltages for each treatment were obtained by Boltzman fitting with the curve of steady-state conductances versus voltages, as described previously (Ilan et al., 1995), and the results are summarized in Table I. In the presence of internal 1.5 μm Ca2+, the Gmax of the Kin channels in abaxial guard cells decreased by 62%, whereas the Gmax of the inward K+ currents in adaxial guard cells was not affected (Table I). The addition of 1 μm ABA in the pipette solution resulted in decreases in the Gmax of 61% and 80% for adaxial and abaxial guard cells, respectively (Table I). The V1/2 values of the Kin channels in abaxial guard cells were shifted to significantly more negative potentials in the presence of internal 1.5 μm Ca2+ (from −118 to −132 mV) or 1 μm ABA (from −118 to −137 mV). However, although the presence of 1 μm ABA in the cytoplasm did not significantly affect V1/2 for adaxial guard cells, the addition of 1.5 μm Ca2+ in the pipette solution resulted in a significant shift of V1/2 toward more positive potentials for adaxial guard cells (Table I). These results demonstrated that the Kin channels in abaxial guard cells were more sensitive to internally applied 1.5 μm Ca2+ or 1 μm ABA. These results may also indicate that there may be different regulation mechanisms of the Kin channels by ABA or Ca2+ in abaxial and adaxial guard cells.
Table I.
Effects of internal Ca2+ or ABA on Gmax and V1/2 of the inward K+ channels in broad bean guard cells
Gmax and V1/2 for each treatment were derived by Boltzman fitting with the curve of steady-state conductances compared with voltages from the individual cell as described previously (Ilan et al., 1995) and are expressed as means ± se. The presented Gmax was normalized and expressed as the conductance per unit cell capacitance (nS pF−1) to account for variations in cell surface area. The numbers in parentheses represent the number of replicates for each treatment.
| Treatment |
Gmax
|
V1/2
|
||
|---|---|---|---|---|
| Adaxial | Abaxial | Adaxial | Abaxial | |
| nS pF−1 | mV | |||
| Control | 0.69 ± 0.04 (9) | 0.84 ± 0.02 (6) | −124 ± 3.2 (9) | −118 ± 2.0 (6) |
| 1.5 μm Ca2+ | 0.70 ± 0.03 (11) | 0.32 ± 0.02 (6)a | −112 ± 2.1 (11)a | −132 ± 4.7 (6)b |
| 1 μm ABA | 0.27 ± 0.03 (14)a | 0.17 ± 0.02 (10)a | −130 ± 3.1 (14) | −137 ± 3.3 (10)a |
P ≤ 0.05.
P ≤ 0.01.
Effects of External ABA on Inward K+ Currents of Abaxial and Adaxial Guard Cell Protoplasts
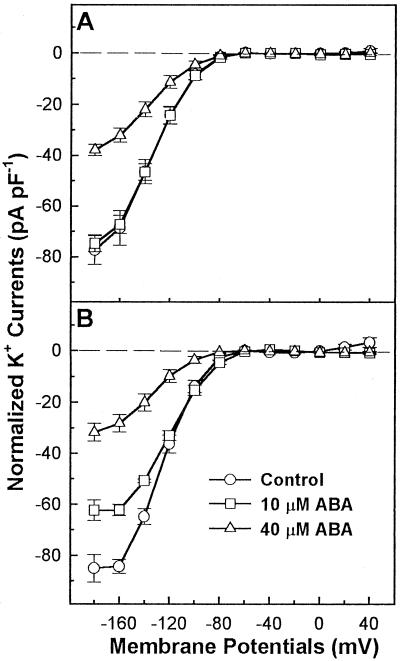
Figure 6 shows current/voltage relations in the presence or absence of ABA in the bath solution. The addition of 40 μm ABA in the bath solution inhibited the inward K+ currents (at both −160 and −180 mV) by 65% in abaxial guard cells and by 50% in adaxial guard cells. In the presence of external 40 μm ABA, Gmax of the Kin channels in adaxial and abaxial guard cells were decreased by 53% and 54%, respectively (Table II). However, the V1/2 for the inward currents of abaxial guard cells was significantly shifted to more negative potentials (from −115 to −128 mV), and the V1/2 for adaxial guard cells remained unchanged (Table II).
Figure 6.
Current/voltage relations of whole-cell inward K+ currents in adaxial (A) and abaxial (B) guard cell protoplasts in the absence or presence of ABA in the bath solution. K+ current amplitudes were expressed as the current per unit cell capacitance to correct for variation resulting from different cell sizes. The number of replicates of whole-cell recordings with adaxial guard cells were 7 (Control), 11 (10 μm ABA), and 9 (40 μm ABA). The replicates of whole-cell recordings with abaxial guard cells were 7 (Control), 7 (10 μm ABA), and 13 (40 μm ABA). Each data point is the mean ± se.
Table II.
Effects of external ABA on Gmax and V1/2 of the inward K+ channels in broad bean guard cells
Gmax and V1/2 for each treatment were derived by Boltzman fitting with the curve of steady-state conductances compared with voltages from the individual cell as described previously (Ilan et al., 1995) and are expressed as means ± se. The presented Gmax was normalized and expressed as the conductance per unit cell capacitance (nS pF−1) to account for variations in cell surface area. The numbers in the parentheses represent the number of replicates for each treatment.
| Treatments |
Gmax
|
V1/2
|
||
|---|---|---|---|---|
| Adaxial | Abaxial | Adaxial | Abaxial | |
| nS pF−1 | mV | |||
| Control | 0.59 ± 0.04 (7) | 0.70 ± 0.05 (7) | −124 ± 3.4 (7) | −115 ± 3.4 (7) |
| 10 μm ABA | 0.60 ± 0.03 (11) | 0.65 ± 0.06 (7) | −116 ± 4.3 (11) | −100 ± 4.2 (7)b |
| 40 μm ABA | 0.28 ± 0.02 (9)a | 0.32 ± 0.01 (13)a | −125 ± 5.8 (9) | −128 ± 4.8 (13)b |
P ≤ 0.05.
P ≤ 0.01.
Although externally applied 10 μm ABA inhibited the inward K+ currents about 35% at more negative potentials than −140 mV in abaxial guard cells, it did not inhibit the inward currents at more positive potentials than −140 mV. Furthermore, the V1/2 for abaxial guard cells in the presence of 10 μm external ABA was shifted toward a more positive potential (Table II). Figure 6B (abaxial guard cells) shows that the current/voltage curves reached the plateau at the most negative voltages (between −160 and −180 mV) in the presence and absence of 10 μm ABA. This could reflect Ca2+ block of these K+ channels (Marten et al., 1991), although this requires further analysis.
Stomatal Conductance of Abaxial and Adaxial Leaf Surfaces under Normal or Water-Stress Conditions
It is known that stomatal movement is strongly regulated by environmental water conditions and that water-stress-induced stomatal closing is mediated in part by ABA and Ca2+ signals (Zhang and Davis, 1989; Tardieu et al., 1992). Therefore, we tested the effects of water stress on abaxial and adaxial stomatal conductances. A 3-d water-stress treatment resulted in 75.4% (from 128.4 ± 10 to 31.6 ± 7.2 mmol m−2 s−1; n = 13) and 62.7% (from 63 ± 7.2 to 25.6 ± 4 mmol m−2 s−1; n = 13) decreases in the stomatal conductances for abaxial and adaxial surfaces, respectively. The net decrease of stomatal conductance was 96.8 mmol m−2 s−1 for the abaxial leaf surface and only 37.4 mmol m−2 s−1 for the adaxial leaf surface. The results demonstrated that abaxial stomata are indeed more sensitive than adaxial stomata to water-stress signals.
DISCUSSION
Stomatal guard cells have been widely used to study signal transduction mechanisms for more than a decade. However, it remains unknown whether there are different signal transduction pathways in the two different types of guard cells (Chasan, 1995). Since ABA and Ca2+ have been demonstrated as important regulators in guard cell signal transduction, in this study we focused on determining whether abaxial and adaxial guard cells respond differently to Ca2+ and ABA.
The results from the epidermal peel experiments (Figs. 1 and 2) clearly demonstrate that externally applied ABA or Ca2+ has much less of an effect on the regulation of adaxial stomatal movement compared with their effects on abaxial stomata. The abaxial stomata were also more sensitive to water-stress treatment compared with adaxial stomata in our gas-exchange experiments. In agreement with these results, the internally applied Ca2+ strongly inhibited inward K+ currents of abaxial guard cells but had essentially no effect on the inward K+ currents in adaxial guard cells (Figs. 3 and 4). These results suggest that the abaxial stomata are more sensitive to Ca2+ or ABA in their movement processes. Since the inward K+ currents in our experiments were activated at membrane potentials more negative than −100 mV, the inhibitory effects of ABA or Ca2+ on the inward currents may account for their effects on stomatal opening. The physiological mechanisms for the differential responses to the externally applied ABA and Ca2+ in the closing process of the two types of stomata remain to be uncovered but may include the involvement of anion channels, Kout channels, or other components. The whole-cell outward K+ currents were not really activated in our recordings. Part of the reason for the low outward K+ currents may be that the highest depolarizing potential was only −40 mV in our experiments. The low outward K+ currents may have also resulted from other unidentified variables such as growth conditions.
Both intracellular and externally facing ABA receptors may exist in guard cells (Assmann, 1994). In our experiments application of ABA directly to the cytosol inhibited inward K+ currents, which is consistent with previous reports showing that one locus of ABA action is internal (Allan et al., 1994; Schwartz et al., 1994). Application of ABA on the cytoplasmic side had similar effects on the inward K+ currents in both abaxial and adaxial guard cells, despite the fact that abaxial guard cells were more sensitive to externally applied ABA in both epidermal peel (Figs. 1 and 2) and patch-clamp (Fig. 6) experiments. There are several possible explanations for this apparent discrepancy. One micromolar ABA applied internally may be a sufficiently high concentration to saturate the ABA response of both cell types, in which case differences in sensitivity would not be detected by this treatment. Alternatively, the two cell types could have equal sensitivity to internal ABA, but the abaxial guard cells may respond to apoplastic ABA more sensitively. This could occur, for example, if there was an ABA transporter on the plasma membrane that was more abundant, or functioned more efficiently, in abaxial guard cells. Although few data are available so far about ABA transporters, Daeter and Hartung (1993) have provided evidence for such a transport molecule functioning in epidermal cells. Under the assumption that at least some of the effects of externally applied ABA are mediated by an ABA receptor on the outside surface of the guard cell (Hartung, 1983; Anderson et al., 1994; Assmann, 1994), such receptors may be either more sensitive or more abundant in abaxial guard cells.
Some studies have suggested that ABA-induced stomatal closure is mediated by an ABA-induced Ca2+ increase in the guard cell cytoplasm (Keller et al., 1989; Schroeder and Hagiwara, 1989; Hedrich et al., 1990; McAinsh et al., 1990; Blatt, 1992), whereas other studies have indicated that ABA does not always induce a cytoplasmic Ca2+ increase in guard cells (Gilroy et al., 1991; Irving et al., 1992; McAinsh et al., 1992). It has been debated for years whether there is only one (MacRobbie, 1992; McAinsh et al., 1992) or two different signal transduction routes for ABA-induced stomatal closure, i.e. Ca2+-dependent and Ca2+-independent pathways (MacRobbie, 1990; Allan et al., 1994). The data presented here demonstrate that ABA significantly regulates the Kin channels in both abaxial and adaxial guard cells but that Ca2+ regulates only the Kin channels in abaxial guard cells. Therefore, we hypothesize that ABA regulates adaxial stomatal movement (or at least adaxial Kin channels) via a Ca2+-independent pathway.
Goh et al. (1995) reported that maximal H+-pumping activities in abaxial and adaxial guard cell protoplasts were almost the same. The data presented in Figures 4–6 show that the normalized whole-cell inward K+ currents in abaxial and adaxial guard cells were also almost the same. Taken together, these results indicate that the distribution or densities of H+ pumps and K+ channels in the plasma membranes of the two types of guard cells may be equal. On the other hand, H+ pumps in adaxial guard cells require a larger number of photons for their activation (Goh et al., 1995), and Kin channels in adaxial guard cells were not regulated by Ca2+ as they were in abaxial guard cells (Fig. 4). These observations indicate that the regulatory mechanisms for K+ channels and H+ pumps in the two types of guard cells may be different.
Possible underlying channel-gating components responsible for the differential effects of Ca2+ and ABA on the Kin channels in abaxial and adaxial guard cells may be partially explained by their different effects on Gmax and V1/2 of the Kin channels in two types of guard cells. Since Gmax is the function of the number of voltage-independent available channels for activation (Ilan et al., 1995), the greater effects of internally applied Ca2+ and ABA on Gmax in abaxial guard cells (Table I) suggest that the greater inhibition of the inward K+ currents in abaxial guard cells by the internally applied Ca2+ and ABA may result from their greater effects on the number of channels available for activation. The internally applied Ca2+ and ABA also resulted in the significant shifts of V1/2 toward more negative potentials for abaxial guard cells (Table I), which indicates a possible decrease in channel-opening probability in abaxial guard cells (Ilan et al., 1995). Single-channel recording analyses will be required to prove this hypothesis.
The differential effects of the externally applied ABA shown in Figure 6 could not be explained by effects of ABA on Gmax and V1/2 (Table II). A possible explanation for this phenomenon is that external ABA results in a voltage-dependent inactivation superimposed on the voltage activation of the Kin channels in abaxial guard cells, as shown in Figure 6B (note that the current/voltage curves reaches the plateau at the most negative voltages between −160 and −180 mV). Therefore, Gmax and V1/2 may not be expected to precisely describe the channel behavior. Further study is required to resolve underlying factors responsible for the differential effects of external ABA on the Kin channels in abaxial and adaxial guard cells.
Based on previous work and the results presented here, it is reasonable to hypothesize that there are different signal transduction pathways for the regulation of abaxial and adaxial stomatal movements, at least for ABA- and Ca2+-mediated effects. Since abaxial stomata play a major role in gas exchange between leaves and the environment, and abaxial stomata are also more sensitive to environmental (e.g. light) and internal (e.g. ABA) stimuli, abaxial stomata or guard cells may be more revealing for signal transduction studies. The separation of abaxial and adaxial guard cells in the isolation process may be critical for studies of guard cell protoplasts and may explain much of the variability in guard cell responses reported in previous studies in which this separation was not achieved.
Abbreviations:
- Gmax
maximal conductance
- Kin and Kout channels
inward- and outward-rectifying K+ channels, respectively
- V1/2
half-activation voltage
Footnotes
This study was supported by a National Outstanding Young Scientist grant (no. 39525003) and a Competitive Research grant (no. 39470359 to W.-H.W.) from the National Science Foundation of China. X.-Q.W. was partially supported by a Ph.D. program grant to Northwestern Agricultural University from the National Educational Commission of China.
LITERATURE CITED
- Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ. Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BE, Ward JM, Schroeder JI. Evidence for an extracellular reception site for abscisic acid in Commelina guard cells. Plant Physiol. 1994;104:1177–1183. doi: 10.1104/pp.104.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Assmann SM. Ins and outs of guard cell ABA receptors. Plant Cell. 1994;6:1187–1190. [Google Scholar]
- Assmann SM, Simoncini L, Schroeder JI. Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature. 1985;318:285–287. [Google Scholar]
- Blatt MR. K+ channels of stomatal guard cells: characteristics of the inward rectifier and its control by pH. J Gen Physiol. 1992;99:615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Grabov A. Signaling gates in abscisic acid-mediated control of guard cell ion channels. Physiol Plant. 1997;100:481–490. [Google Scholar]
- Blatt MR, Thiel G, Trentham DR. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-triphosphate. Nature. 1990;346:766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Bookman RJ, Lin NF, Schweizer SE, Nowycky M. Single cell assays of excitation-secretion coupling. Ann NY Acad Sci. 1991;635:352–364. doi: 10.1111/j.1749-6632.1991.tb36504.x. [DOI] [PubMed] [Google Scholar]
- Chasan R. New openings into stomata. Plant Cell. 1995;7:1113–1115. [Google Scholar]
- Daeter W, Hartung W. The permeability of the epidermal cell plasma membrane of barley leaves to abscisic acid. Planta. 1993;191:41–47. [Google Scholar]
- De Silva DLR, Cox RC, Hetherington AM, Mansfield TA. The role of abscisic acid and calcium in determining the behavior of adaxial and abaxial stomata. New Phytol. 1986;104:41–51. doi: 10.1111/j.1469-8137.1986.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot KA, Assmann SM. Whole-cell K+ current across the plasma membrane of guard cells from a grass: Zea mays. Planta. 1992;186:282–293. doi: 10.1007/BF00196258. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewavas AJ. Role of calcium in signal transduction of Commelina guard cells. Plant Cell. 1991;3:333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh CH, Oku T, Shimazaki K. Properties of proton pumping in response to blue light and fusicoccin in guard cell protoplasts isolated from adaxial epidermis of Vicia leaves. Plant Physiol. 1995;109:187–194. doi: 10.1104/pp.109.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pfluegers Arch Eur J Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartung W. The site of action of abscisic acid at the guard cell plasmalemma of Valerianella locusta. Plant Cell Environ. 1983;6:427–428. [Google Scholar]
- Hedrich R, Busch H, Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Schroeder JI. The physiology of ion channels and electrogenic pumps in higher plant cells. Annu Rev Plant Physiol. 1989;40:539–569. [Google Scholar]
- Ilan N, Moran N, Schwartz A. The role of potassium channels in the temperature control of stomatal aperture. Plant Physiol. 1995;108:1161–1170. doi: 10.1104/pp.108.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc Natl Acad Sci USA. 1992;89:1790–1794. doi: 10.1073/pnas.89.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns EV, Assman SM. The guard cell-environment connection. Plant Physiol. 1993;102:711–715. doi: 10.1104/pp.102.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller BU, Hedrich R, Raschke K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature. 1989;341:450–453. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WB, Esser JE, Schroeder JI. Effects of cytosolic calcium and limited, possible dual, effects of G protein modulators on guard cell inward potassium channels. Plant J. 1995;8:479–489. [Google Scholar]
- Kruse T, Tallman G, Zeiger E. Isolation of guard cell proplasts from mechanically prepared epidermis of Vicia faba leaves. Plant Physiol. 1989;90:1382–1386. doi: 10.1104/pp.90.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtri-Chlieh F, MacRobbie EAC. Role of calcium in the modulation of Vicia guard cell potassium channel channels by abscisic acid: a patch-clamp study. J Membr Biol. 1994;137:99–107. doi: 10.1007/BF00233479. [DOI] [PubMed] [Google Scholar]
- Lu Z. The sensitivity of adaxial and abaxial stomatal resistance in wheat leaf to soil water stress. Acta Phytophysiol Sin. 1988;14:223–227. [Google Scholar]
- Lu Z, Quiñones MA, Zeiger E. Abaxial and adaxial stomata from pima cotton (Gossypium barbadense L.) differ in their pigment content and sensitivity to light quality. Plant Cell Environ. 1993;16:851–858. [Google Scholar]
- Luan S, Li W, Rusnak F, Assmann SM, Schreiber SL. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proc Natl Acad Sci USA. 1993;90:2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC. Calcium-dependent and calcium-independent events in the initiation of stomatal closure by abscisic acid. Proc R Soc Lond B Biol Sci. 1990;241:214–219. [Google Scholar]
- MacRobbie EAC. Calcium and ABA-induced stomatal closure. Proc R Soc Lond B Biol Sci. 1992;338:5–18. [Google Scholar]
- Marten I, Lohse G, Hedrich R. Plant growth hormones control voltage-dependent activity of anion channels in plasma membrane of guard cells. Nature. 1991;353:758–762. [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature. 1990;343:186–188. [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Visualizing changes in cytosolic-free Ca2+ during the response of stomatal guard cells to abscisic acid. Plant Cell. 1992;4:1113–1122. doi: 10.1105/tpc.4.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Calcium ions as second messengers in guard cell signal transduction. Physiol Plant. 1997;100:16–29. [Google Scholar]
- Pemadasa MA. Abaxial and adaxial stomatal responses to light of different wavelengths and to phenylacetic acid on isolated epidermis of Commelina communis L. J Exp Bot. 1982;33:92–99. [Google Scholar]
- Schroeder JI, Fang HH. Inward-rectifying K+ channels in guard cells provide a mechanism for low-affinity K+ uptake. Plant Physiol. 1991;88:11583–11587. doi: 10.1073/pnas.88.24.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Schwartz A, Wu WH, Tucker EB, Assmann SM. Inhibition of inward K+ channels and stomatal response by abscisic acid: an intracellular locus of phytochrome action. Proc Natl Acad Sci USA. 1994;91:4019–4023. doi: 10.1073/pnas.91.9.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K-I, Iino M, Zeiger E. Blue light-dependent proton extrusion by guard cell protoplasts of Vicia faba. Nature. 1986;319:324–326. [Google Scholar]
- Tardieu F, Zhang J, Katerji N, Bethenod O, Palmer S, Davies WJ. Xylem ABA controls the stomatal conductances of field grown maize subjected to soil compaction or soil drying. Plant Cell Environ. 1992;15:193–197. [Google Scholar]
- Travis AJ, Mansfield TA. Light saturation of stomatal opening on the adaxial and abaxial epidermis of Commelina communis. J Exp Bot. 1981;32:1169–1179. [Google Scholar]
- Willmer C, Fricker M. The distribution of stomata. In: Willmer C, Fricker M, editors. Stomata. London: Chapman & Hall; 1996a. pp. 18–19. [Google Scholar]
- Willmer C, Fricker M. Stomatal responses to environmental factors. In: Willmer C, Fricker M, editors. Stomata. London: Chapman & Hall; 1996b. pp. 126–191. [Google Scholar]
- Zeiger E, Farquhar GD, Cowan IR. Stomatal Function. Palo Alto, CA: Stanford University Press; 1987. [Google Scholar]
- Zhang J, Davies WJ. Sequential responses of whole plant water relations towards prolonged soil drying and the mediation by xylem sap ABA concentrations in the regulation of stomatal behavior of sunflower plants. New Phytol. 1989;113:167–174. [Google Scholar]