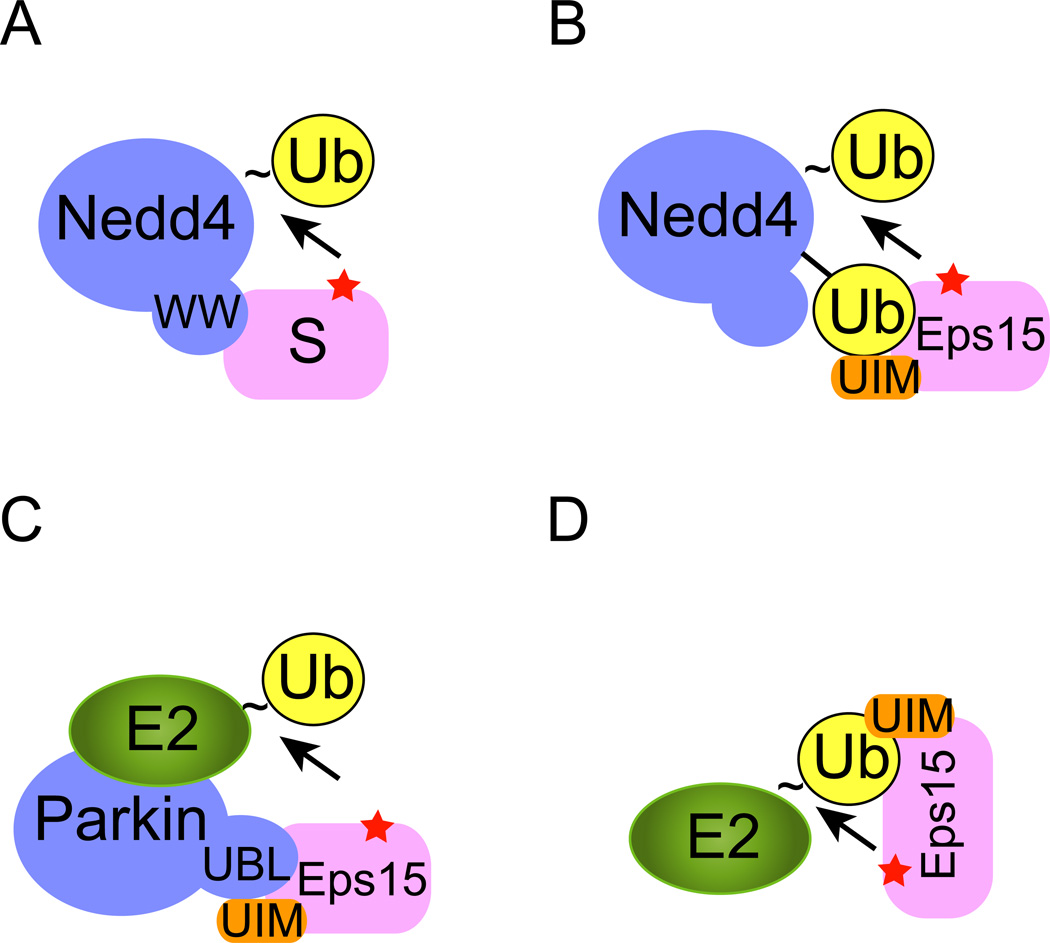
Figure 1. Monoubiquitination of endocytic proteins.
To initiate ubiquitination, a ubiquitin acceptor (usually a Lys residue) in the substrate (red star) needs to be positioned in proximity to an ubiquitin carrier that contains a thioester-linked (indicated by “~”) ubiquitin at its active site. The ubiquitin carrier can be either an E2 or a HECT domain E3 such as Nedd4. A, The HECT domain E3 Nedd4 contains several WW domains that directly recognize a PPXY motif in the substrate (S), leading to the transfer of ubiquitin from Nedd4 to the substrate. B, C, D, Coupled monoubiquitination. B, Substrate recruitment is mediated by interactions of a UBD domain in the substrate (e.g. UIM in Eps15) with a ubiquitin moiety attached to Nedd4. C, The Parkin E3 ligase uses its UBL domain to recruit Eps15. D, An E3 independent mechanism of monoubiquitination. In this case, the UIM domain in Eps15 directly binds the active site-linked ubiquitin on an E2 to mediate ubiquitin transfer in the absence of an E3. Note that a color version of the figure is available online.