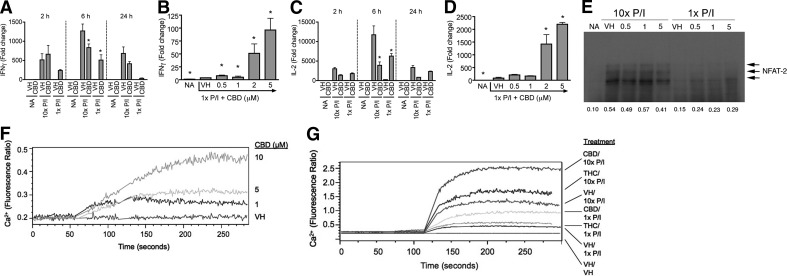
Figure 5. Cannabinoids differentially regulated IL-2 and IFN-γ steady-state mRNA expression by modulating nuclear translocation of NFAT2 and increased intracellular calcium regardless of activation level.
(A–D) B6C3F1 splenocytes (5×106 cells) were treated with CBD (0.5–5 μM) or VH (0.1% ethanol) for 30 min and then activated with P/I (10× P/I 40 nM/0.5 μM, 10× P/I; or 1× P/I 4 nM/0.05 μM, 1× P/I) for various times. Total RNA was isolated and reverse-transcribed, and real-time PCR was performed for IFN-γ (A and B) and IL-2 (C and D). Data are expressed as the mean fold change ± se of triplicate cultures. *P < 0.05 as compared with respective VH. (E) B6C3F1 splenocytes (5×106 cells) were treated with 0.5–5 μM CBD or VH (0.1% ethanol) for 30 min, followed by activation with 1× or 10× P/I for 30 min. Nuclear protein was isolated and loaded into an 8% SDS-PAGE gel. NFAT2 is detected as multiple bands in the 100- to 130-kDa range. (F and G) B6C3F1 splenocytes (2×107 cells) were incubated with Fura Red and Fluo-3 for 30–60 min in the dark. Cells were then analyzed by flow cytometry, during which time they received [CBD (1–10 μM) in F] or [THC (10 μM), CBD (5 μM) or VH (0.1% ethanol) for 1 min followed by 1× P/I, 10× P/I, or VH (0.1% DMSO) for 3 additional min in G]. Results are representative of at least two separate experiments.