FIGURE 9.
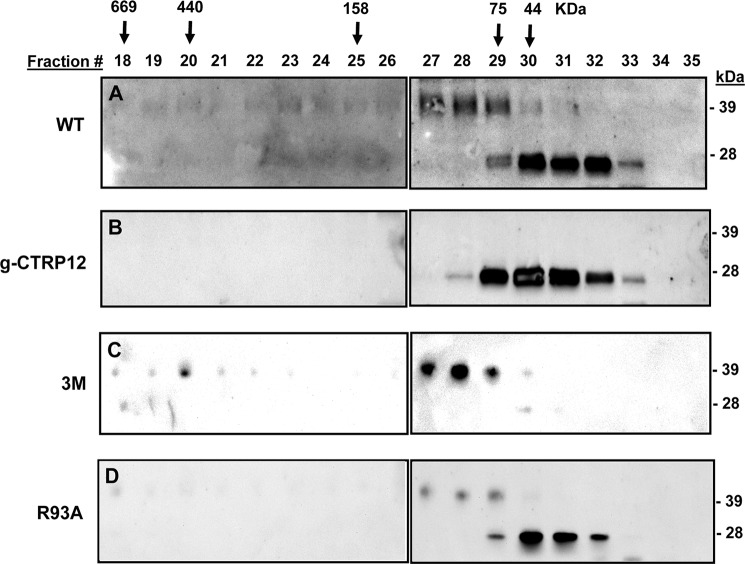
fCTRP12 and gCTRP12 differ in oligomeric structure. Conditioned media from transfected HEK 293T cells containing wild-type (WT) CTRP12 (A), gCTRP12 (B), the 3M mutant (C), or the R93A mutant (D) protein were subjected to gel filtration chromatographic analysis. Arrows with the molecular mass markers of 669, 440, 158, 75, and 44 kDa correspond to the peak elution fraction of molecular standards thyroglobulin, ferritin, aldolase, conalbumin, and ovalbumin, respectively. WT CTRP12 consists of two distinct oligomeric forms: the higher molecular mass (HMM; ∼120 kDa or larger) oligomers composed of full-length protein, and the lower molecular mass (LMM; ∼45 kDa) oligomers consisted of the cleaved gCTRP12 isoform. Recombinant gCTRP12 and the R93A mutant consisted predominantly of the LMM oligomeric form (B and D), whereas the 3M mutant consisted predominantly of the HMM oligomeric form (C).