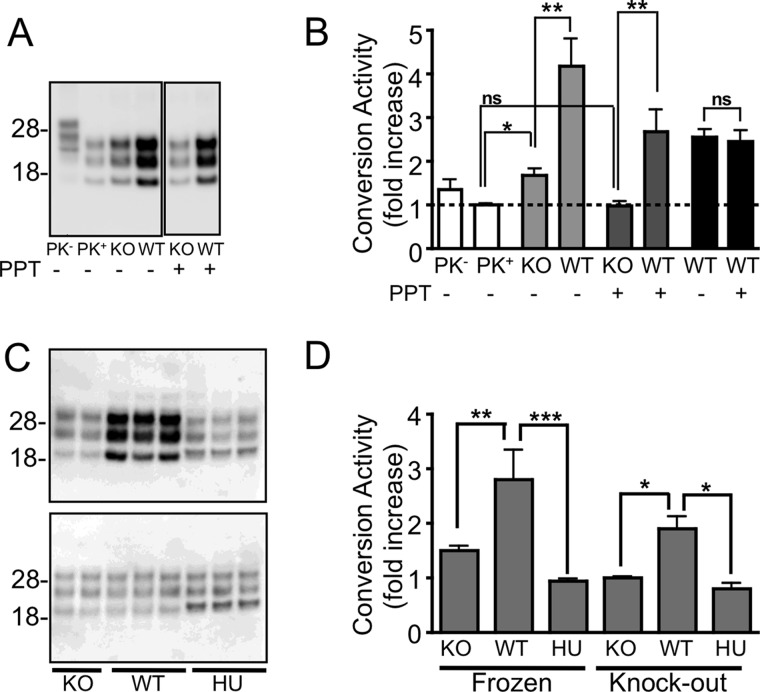
FIGURE 1.
Species-specific formation of PrPres using the insoluble protease-resistant core of PrPSc. A and B, Western immunoblotting (A) and quantitation (B) of in vitro conversion activity assay using an M1000 prion-infected brain homogenate with (+) and without (−) PPT. The small but significant (p < 0.01; t test) increase in PrPres generated in the presence of a Prnp−/− (KO) brain homogenate from untreated PrPSc (light gray bars) relative to the same PrPSc seed diluted in DPBS (clear bars, PK+) was not seen after PPT (dark gray bars) but could form PrPres from a wild type mouse brain homogenate (*, p < 0.05; **, p < 0.01, t test). The conversion activity of PrPSc with and without PPT did not significantly differ (p = 0.8015; two-tailed t test) when the -fold increase was calculated relative to their respective knock-out controls (black bars), indicating that PPT did not affect the ability of PrPSc to seed a conversion reaction. C, PPT of a M1000 prion-infected brain homogenate allowing increased production of PrPres (upper blot) from a substrate derived from wild type mouse brain (WT) but not from a Prnp−/− (KO) or human brain (HU) homogenate when incubated at 37 °C. No increase in PrPres was detected from matching reactions frozen at −80 °C (lower blot). D, quantitation of conversion activity as a -fold increase over the frozen sample or relative to the KO substrate showing significant conversion activity by substrate containing murine PrPC. *, p < 0.05; **, p < 0.01; ***, p < 0.001; one-way analysis of variance with Tukey's Multiple Comparison Test. Error bars, S.E.