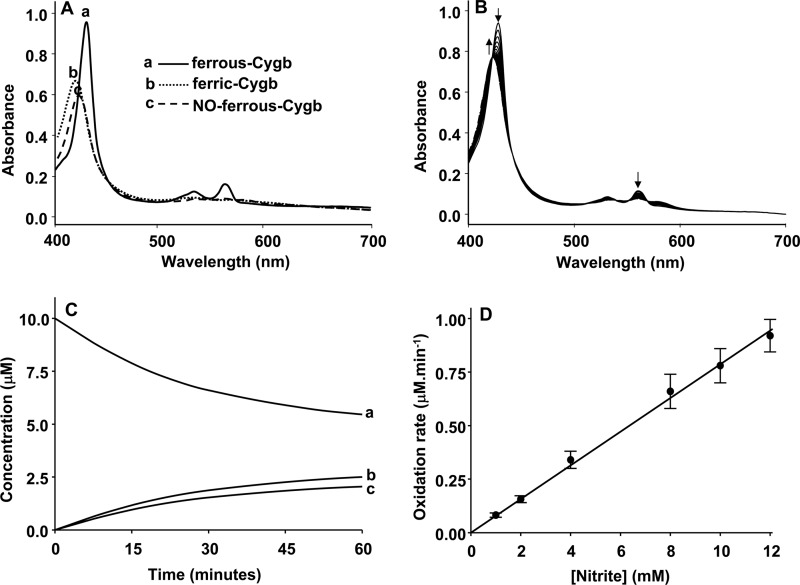
FIGURE 2.
Kinetics of cytoglobin oxidation by nitrite at pH 7.0 under anaerobic conditions. A, standard visible absorption spectra of deoxycytoglobin of ferrous-Cygb (10 μm) (a), ferric-Cygb (10 μm) (b), and NO-ferrous-Cygb (10 μm) in phosphate buffer (pH 7.0) (c). Measurements were made at room temperature under anaerobic conditions as described under “Experimental Procedures.” B, reaction of Cygb (10 μm) with nitrite (2 mm) in phosphate buffer (pH 7.0) under anaerobic conditions, and the visible absorbance spectra of the reaction mixture was measured every 4 min. C, time-dependent concentration change of ferrous-Cygb (a), ferric-Cygb (b), and NO-ferrous-Cygb (c) spectra in the reaction mixture of B. D, nitrite (1–12 mm) concentration-dependent Cygb (10 μm) oxidation rates in phosphate buffer (pH 7.0) under anaerobic conditions. The points show the measured experimental values from the mean of three repeated measurements ± S.D., and lines show the linear regression fit of the data points with correlation coefficient γ2 >0.98.