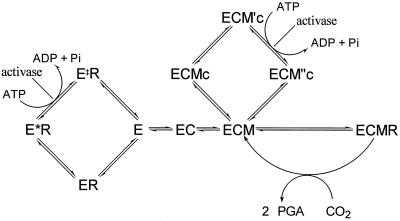
Figure 5.
The pathways involved in the regulation of Rubisco activity. Rubisco active sites (E) can bind sequentially an activator CO2 (C) and a Mg2+ ion (M) to form the active ternary complex ECM. This form can then bind substrate RuBP (R) and catalyze either carboxylation or oxygenation. Rubisco sites may be inactivated by either of two mechanisms: the binding of R to the noncarbamylated E and the binding of CA1P (c) to ECM. The E form binds R in two stages: an initial loose association occurs (ER), followed by a slow conformational change to produce the higher affinity form E*R. The binding of c to ECM also probably takes two steps, initially forming ECMc and finally forming ECM′c. Activase interacts with forms of Rubisco associated with sugar phosphates (such as RuBP and CA1P) in a manner that reduces the affinity of Rubisco for the ligand. ECM′c is converted into ECM“c, which readily dissociates to produce ECM, and E*R is converted into E‡R, which readily dissociates to produce E. There is no evidence that either E‡R is different from ER or ECM“c is different from ECMc. We differentiate between them because they arise from different processes and, therefore, may be different. The products of activase catalysis (E‡R and ECM“c) are not necessarily homologous. PGA, Phosphoglyceraldehyde.