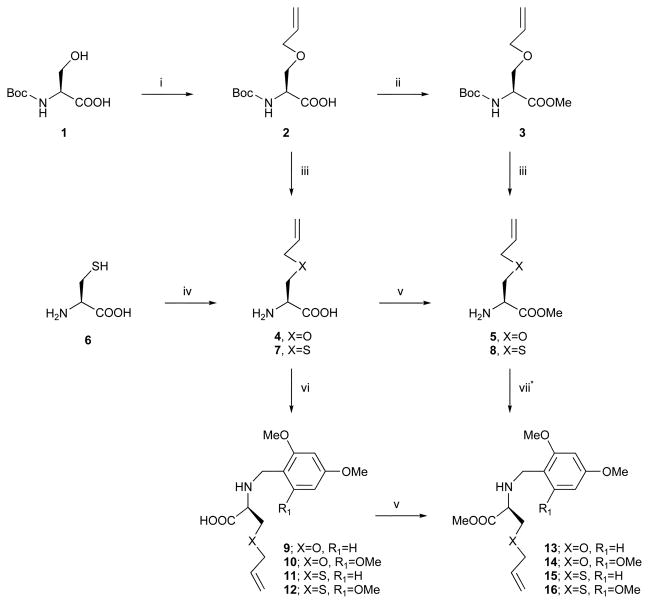
Scheme 1.
Preparation of protected Ser and Cys derivatives. Reagents and conditions: (i) NaH, allylbromide, DMF, rt, 94%; (ii) TMSCHN2, Et2 O–MeOH 1: 1, rt, 78%; (iii) TFA, CH2 Cl2, rt, 93% (5); (iv) EtONa, allylbromide, EtOH, rt, 76%; (v) TMSCHN2, Et2 O–CH2 Cl2 –MeOH 1: 5: 5, rt, 33% (13), 32% (14), 55% (15), 22% (16); (vi) 2,4-dimethoxybenzaldehyde or 2,4,6-trimethoxybenzaldehyde, NaBH3 CN, MeOH, rt, 53% (9), 53% (10), 41% (11), 41% (12); (vii) 2,4-dimethoxybenzaldehyde or 2,4,6-trimethoxybenzaldehyde, NaBH(OAc)3, CH2 Cl2, rt, 85% (13), 82% (14), 74% (15), 58% (16), *partial racemization was obtained in this reaction.