Abstract
Photo-oxidations of hydrogen-bonded phenols using excited state polyarenes are described, to derive fundamental understanding of multiple-site concerted proton-electron transfer reactions (MS-CPET). Experiments have examined phenol-bases having −CPh2NH2, −Py, and −CH2Py groups ortho to the phenol hydroxyl group and tert-butyl groups in the 4,6-positions for stability (HOAr-NH2, HOAr-Py, and HOAr-CH2Py, respectively; Py = pyridyl; Ph = phenyl). The photo-oxidations proceed by intramolecular proton transfer from the phenol to the pendent base concerted with electron transfer to the excited polyarene. For comparison, 2,4,6-tBu3C6H2OH, a phenol without a pendent base and tert-butyl groups in the 2,4,6-positions, has also been examined. Many of these bimolecular reactions are fast, with rate constants near the diffusion limit. Combining the photochemical kCPET values with those from prior thermal stopped-flow kinetic studies gives datasets for the oxidations of HOAr-NH2 and of HOAr-CH2Py that span over 107 in kCPET and nearly 0.9 eV in driving force (ΔGo′). Plots of log(kCPET) vs. ΔGo′ define a single Marcus parabola in each case, each including both excited state anthracenes and ground state aminium radical cations. These two datasets are thus well described by semi-classical Marcus theory, providing a strong validation of the use of this theory for MS-CPET. The parabolas give λCPET ≅ 1.15–1.2 eV and Hab ≅ 20–30 cm−1. These experiments represent the most direct measurements of Hab for MS-CPET reactions to date. Although rate constants are available only up to the diffusion limit, the parabolas clearly peak well below the adiabatic limit of ca. 6 × 1012 s−1. Thus, this is a very clear demonstration that the reactions are non-adiabatic. The non-adiabatic character slows the reactions by a factor of ~45. Results for the oxidation of HOAr-Py, in which the phenol and base are conjugated, and for oxidation of 2,4,6-tBu3C6H2OH, which lacks a base, show that both have substantially lower λ and larger pre-exponential terms. The implications of these results for MS-CPET reactions are discussed.
Introduction
Proton-coupled electron transfer (PCET) reactions are integral to a wide range of processes, including oxygen production and reduction in photosynthesis, mitochondria and fuel cells, catalytic nitrogen fixation, and hydrocarbon oxidations. Because of this widespread importance, PCET has been studied in many contexts via experiments, computations, and new theoretical approaches.1–4 Of most interest are reactions in which a proton and an electron transfer in a single kinetic step, termed concerted proton-electron transfer (CPET) reactions. Despite the widespread attention on these reactions, questions remain about the conceptual and practical models that should be used. The key question addressed here is whether these reactions should be treated as adiabatic or non-adiabatic. Most chemical reactions are treated as adiabatic processes, occurring on a single free energy surface. Electron transfer reactions, however, are typically considered to be non-adiabatic transitions from a reactant surface to a product surface. This non-adiabatic nature means that they have maximum reaction rates below ca. 6 × 1012 s−1 under barrier-less conditions, as described below. Most of the theoretical treatments of CPET start from non-adiabatic models, as described in a recent special issue of Chemical Reviews.1 The same issue of Chem. Rev. contains an extensive review of computational (typically DFT) studies of PCET reactions, which almost invariably assume adiabatic reactivity.5 It has been proposed that the extent of non-adiabaticity in these processes is the most direct distinction between different types of reactions under the PCET umbrella.6,7 This proposal comes mostly from theorists, because it is experimentally challenging to evaluate non-adiabatic character in a reaction.
To probe fundamental questions in PCET, we and others have used model systems incorporating phenols. These studies are also relevant to biological energy production, biosynthesis, and antioxidant activity.8 Biological tyrosine oxidations involve proton transfer and form the neutral tyrosyl radical. The most notable example is the oxidation of YZ in photosystem II that occurs with proton movement to a hydrogen-bonded histidine base (H-bonds to aspartate, glutamate, and lysine residues are also biologically relevant).8–10 Linschitz and coworkers were the first to study phenol oxidations in which the proton transfers to a base concerted with electron transfer to a separate oxidant, which can be termed multiple-site concerted proton-electron transfer (MS-CPET).11,12 These studies have been extended in many ways, including thermal, photochemical, and electro-chemical oxidations.10–42 Our group21–24,29 and others19,20,25–27,41 have examined intramolecular H-bonded phenols, as mimics of the YZ-His site in PSII,10,25 and Hammarström, Meyer, Nocera, and others have tethered phenol or tyrosine derivatives to photo-oxidants.10,18,19,31–40
These phenol MS-CPET reactions have been analyzed with various levels of theory. A few papers have applied versions of Hammes-Schiffer's multistate continuum theory, although this is challenging and simplifications usually have to be applied because many of the needed parameters are not easily accessible.22,32,43–49 More typically, versions of the semi-classical Marcus theory of electron transfer (ET) have been used (eq 1).19,21–23,28,29,33,42,50–54 This has also been applied to electrochemical CPET, via the Marcus-Hush-Levich formalism.15–17,20,27,41,55–57
| (1) |
Equation 1 gives the rate constant in terms of the corrected reaction driving force ΔGo′, the intrinsic barrier λ, an Hab coupling parameter, the temperature T, and the Boltzmann and Planck constants. Hab is the electronic coupling between the reactant and product diabatic (non-interacting) states, and it is the quantitative measure of non-adiabaticity. At the crossing point of the diabatic states, this coupling causes a mixing of two diabatic states into two adiabatic states separated by 2Hab. When Hab is more than kBT (207 cm−1 at 25 °C), the system predominantly stays on the lower surface and can be treated as an adiabatic reaction, as occurring on a single surface.58 As noted above, this is the typical situation for most chemical reactions. When the coupling is smaller the reaction is non-adiabatic and there is only a small probability that the reactants will progress to products when they have the nuclear configuration of the crossing region.
Here we determine the extent of adiabaticity in MS-CPET reactions of hydrogen-bonded phenols by mapping the Marcus parabola of rate constant versus driving force. At the top of this parabola the free energy barrier is zero because the driving force is the same magnitude as the reorganization energy (ΔGo′ = −λ). The rate constant at the top of the parabola is a direct measure of the non-adiabaticity and Hab. This is the approach that Gray et al. have used to measure the distance dependence of Hab and electron transfer rate constants.59,60 Adiabatic reactions have maximum first-order rate constants of ~6 × 1012 s−1 (kBT/h, the Eyring prefactor).58 Equation 1 gives a 6 × 1012 s−1 prefactor at T = 298 K with λ = 1.2 eV (as appropriate for the compounds here) when Hab = 160 cm−1. Conceptually, when the top of the parabola is close to k = 6 × 1012 s−1 then the reaction can be considered adiabatic, while lower peak rate constants indicate the importance of the non-adiabatic character. For instance, the reactions of excited state RuII-diimine complexes with cytochrome c are significantly non-adiabatic, with Hab ~ 1 cm−1 and ET parabolas that peak at k = 3×108 s−1.61
Reported here are rate constants for MS-CPET oxidation of hydrogen-bonded phenols from time-resolved fluorescence quenching experiments, employing excited state polyarenes as photo-oxidants (Scheme 1). Combining these data with previously reported stopped-flow measurements from our laboratory21,23,24,62 gives a set of rate constants from 103 M−1 s−1 to the diffusion limit of 1010.2 M−1 s−1, with driving forces ΔGo′ from +0.2 to nearly −2 eV. This dataset describes a parabola truncated by the diffusion limit, and is well-analyzed using the semi-classical Marcus model. These experiments provide the most direct measure to date of the non-adiabaticity of these biologically and technologically relevant transformations.
Scheme 1.
(A) Kinetic scheme for phenol-base MS-CPET. (B) Phenols, Photo-oxidants, and Oxidants.
Experimental
I. General
Unless otherwise noted, all reagents were purchased from Aldrich. Acetonitrile (HPLC grade) was purchased from EMD (results were identical when `low water brand' acetonitrile from Burdick and Jackson was used). Deuterated solvents were purchased from Cambridge Isotope Laboratories. HOAr-NH2,24HOAr-Py,63 and HOAr-CH2Py21 were prepared by literature methods, and 2,4,6-tri-tert-butylphenol (2,4,6-tBu3C6H2OH) was purchased from Aldrich and recrystallized from ethanol.
II. Physical Measurements
1H NMR spectra were recorded at ambient temperatures on Bruker AF300, AV300, AV301, DRX499 or AV500 spectrometers. UV-vis spectra were collected on an Hewlett Packard 8453 diode array spectrophotometer. Steady-state fluorescence spectra were collected on a Perkin-Elmer LS-50B instrument.
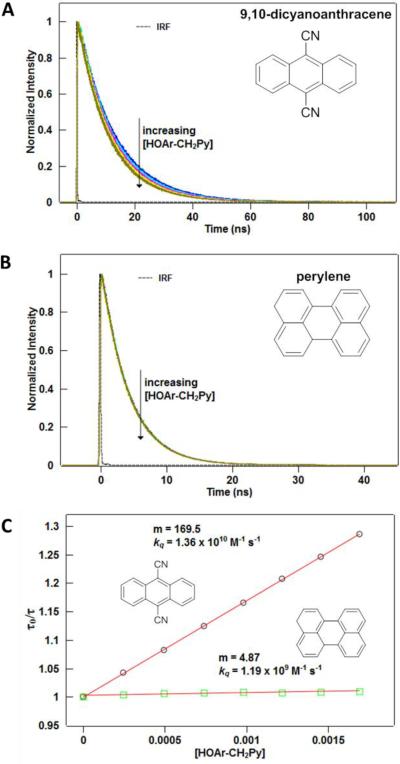
Time-Correlated Single Photon Counting (TCSPC) was carried out using a PicoQuant FluoTime 100 time-resolved fluorescence spectrometer with the PicoHarp 300 stand-alone photon counting module in the Photonics Center at the University of Washington. Picosecond pulsed diode lasers (wavelengths: 375, 405, and 470 nm) controlled by the PDL 800-D driver, produce excitation pulses as short as 70 ps (FWHM) at a repetition rate of 80 MHz. A series of filters were employed to block the scatter of the excitation source. The acquisition counts per second was held at a value of ~105 s−1, much less than the fluorescence lifetime of the fluorophores (~108 s−1 for most of the fluorophores used herein) to avoid instrumental artifacts. In general, the acquisition time was 5–10 minutes. A typical 2 mL sample consisted of 10 μM of the fluorophore in acetonitrile with 1–3% MeOH or MeOD. Bimolecular kinetics were measured by adding in a known amount of the phenol-base of interest (the quencher), measuring the kinetics, and adding an additional three or four aliquots of the phenol in order to construct a Stern-Volmer plot (see Figure 1 and Supporting Information (SI)). The quencher was typically added in 50–100 μL aliquots such that the final quencher concentration after three or four additions was 2–10 mM. Larger final concentrations of quencher were necessary for slower reactions with small fluorescence lifetimes. Three Stern-Volmer quenching rates were measured for each fluorophore/quencher combination to gauge the error of the measurement. Fits of the time-resolved fluorescence data to monoexponential decays were carried out using the FluoFit software package,64 in which the instrument response function (IRF, obtained by scattering the excitation source into the detector using a water/Ludox suspension) was convoluted with an exponential decay to fit the data.
Figure 1.
Examples of HOAr-CH2Py quenching the fluorescence of (A) 9,10-dicyanoanthracene and (B) perylene. The instrument response function (IRF), which indicates the temporal width of the excitation pulse, in shown in both cases. (C) Stern-Volmer plots for these reactions, which allow calculation of the quenching rate constant (kq) from the slope (m).
Examples of time-resolved fluorescence datasets and Stern-Volmer plots for this system can be found in Figure 1. Kinetic isotope effects were measured by adding an excess of MeOD (typically ~1–3% of the total volume) to the acetonitrile solutions and measuring the fluorescence lifetime as a function of added quencher as described above. As a control, MeOH was added to measure kH, with rate constants being within the uncertainty of measurements in pure acetonitrile.
III. Calculations
All DFT calculations were carried out in Gaussian 0965 on the Stuart Cluster at the chemistry department at the University of Washington. Calculations were done at the UB3LYP/6-311++G(d,p) level with an acetonitrile PCM model. The self-exchange innersphere reorganization energy for the polyarenes, λi, was calculated according to the 4-point model of Nelsen.66,67 The value of λi for [N(tol)3]•+ was taken to be characteristic for all [N(Ar)3]•+, and thus no other 4-point calculations were carried out for these oxidants. All ground state calculations were shown to have no imaginary frequencies (using the freq=noraman keyword), except for that of 9-methylanthracene which displayed an imaginary methyl rotational mode. As a comparison, several time-dependent calculations of excited state energies were carried out as described in the Supporting Information (SI).
Results and Discussion
I. Rate Constants and Driving Forces
The oxidations of phenols by excited state polyarenes were monitored by time-resolved fluorescence quenching. Addition of increasing amounts of the phenol-base compounds decreases the lifetime of the fluorophore (Figure 1). In general, reactions proceeding with kobs > 108 M−1 s−1 provided well-behaved monoexponential fluorescence decays. The changes in lifetime vary linearly with phenol concentration, giving quenching rate constants (kobs) by standard Stern-Volmer analysis (Figure 1C, Table 1, and see SI). Correlation values for the Stern-Volmer plots were generally near R2 = 1, although slower reactions did suffer a larger relative error.
Table 1.
Reaction driving force, observed quenching rate, and various corrections for three different H-bonded phenols with both polyarene photo-oxidants and aminium oxidants.
| HOAr-NH2 | ΔG°CPET (eV) a | w (eV) b | ΔG°′CPET (eV) c | kobs (M−1s−1) d | kCPET (s−1) e |
|---|---|---|---|---|---|
| [TPP](BF4) g | −1.76 | 0.00 | −1.76 | 1.3 ± 0.2 × 1010 | ≥ kd f |
| 9,10-dicyanoanthracene | −1.15 | −0.03 | −1.18 | 1.1 ± 0.1 × 1010 | ≥ kd f |
| 9-cyanoanthracene | −0.63 | −0.04 | −0.67 | 5.4 ± 1.0 × 109 | 8.3 × 109 |
| anthracene | −0.59 | −0.04 | −0.63 | 2.7 ± 0.2 × 109 | 3.2 × 109 |
| fluoranthene | −0.55 | −0.04 | −0.59 | 9.8 ± 1.0 × 108 | 1.1 × 109 |
| 9-phenylanthracene | −0.52 | −0.03 | −0.55 | 1.8 ±0.1 × 109 | 2.0 × 109 |
| benzo[b]triphenylene | −0.47 | −0.04 | −0.51 | 9.8 ± 5.0 × 108 | 1.0 × 109 |
| 9,10-diphenylanthracene | −0.46 | −0.03 | −0.49 | 7.6 ± 1.8 × 108 | 8.0 × 108 |
| 9-methylanthracene | −0.44 | −0.04 | −0.48 | 1.3 ± 0.2 × 109 | 1.4 × 109 |
| perylene | −0.39 | −0.04 | −0.43 | 1.1 ± 0.1 × 109 | 1.2 × 109 |
| benzo[c]phenanthrene | −0.36 | −0.04 | −0.40 | 2.0 ± 0.9 × 108 | 2.0 × 108 |
| tetracene | −0.29 | −0.03 | −0.32 | 8.0 ± 1.1 × 108 | 8.4 × 108 |
| benzo[a]pyrene | −0.21 | −0.03 | −0.24 | 6.2 ± 1.5 × 108 | 6.4 × 108 |
| [N(p-C6H4Br)3]●+ | −0.31 | 0.00 | −0.31 | 4 ± 2 × 107 j | 4 × 107 |
| [N(p-C6H4OMe)(p-C6H4Br)2]●+ | −0.12 | 0.00 | −0.12 | 8 ± 1 × 105 j | 8 × 105 |
| [N(tol)3]●+ h | −0.02 | 0.00 | −0.02 | 1.1 ± 0.2 × 105 j | 1.2 × 105 |
| [N(p-C6H4OMe)2(p-C6H4Br)]●+ | 0.04 | 0.00 | 0.04 | 2.7 ± 0.3 × 104 j | 2.7 × 104 |
| [MPT]●+ i | 0.04 | 0.00 | 0.04 | 3.2 ± 0.3 × 104 j | 3.2 × 104 |
| [N(p-C6H4OMe)3]●+ | 0.20 | 0.00 | 0.20 | 1.1 ± 0.1 × 103 j | 1.1 × 103 |
| HOAr-Py | ΔG°CPET (eV) a | w (eV) b | ΔG°′CPET (eV) c | kobs (M−1s−1) d | kCPET (s−1) e |
|---|---|---|---|---|---|
| [TPP](BF4) g | −1.55 | 0.00 | −1.55 | 1.5 ± 0.2 × 1010 | ≥ kd f |
| 9,10-dicyanoanthracene | −0.94 | −0.03 | −0.97 | 1.4 ± 0.1 × 1010 | ≥ kd f |
| 9-cyanoanthracene | −0.42 | −0.04 | −0.46 | 9.1 ± 1.1 × 109 | ≥ kd f |
| anthracene | −0.38 | −0.04 | −0.42 | 1.2 ± 0.2 × 1010 | ≥ kd f |
| fluoranthene | −0.34 | −0.04 | −0.38 | 5.4 ± 0.16 × 109 | 8.2 × 109 |
| 9-phenylanthracene | −0.31 | −0.03 | −0.34 | 1.1 ± 0.09 × 1010 | ≥ kd f |
| 9,10-diphenylanthracene | −0.25 | −0.03 | −0.28 | 7.4 ± 0.79 × 109 | ≥ kd f |
| 9-methylanthracene | −0.22 | −0.04 | −0.26 | 1.1 ± 0.05 × 1010 | ≥ kd f |
| perylene | −0.18 | −0.04 | −0.22 | 7.4 ± 0.49 × 109 | ≥ kd f |
| benzo[c]phenanthrene | −0.15 | −0.04 | −0.19 | 6.0 ± 0.10 × 109 | 9.6 × 109 |
| tetracene | −0.08 | −0.03 | −0.11 | 3.9 ± 0.49 × 109 | 5.2 × 109 |
| benzo[a]pyrene | 0.00 | −0.03 | −0.03 | 6.0 ± 0.95 × 109 | 9.7 × 109 |
| HOAr-CH2Py | ΔG°CPET (eV) a | w (eV) b | ΔG°′CPET (eV) c | kobs (M−1s−1) d | kCPET (s−1) e |
|---|---|---|---|---|---|
| [TPP](BF4) g | −1.69 | 0.00 | −1.69 | 1.4 ± 0.2 × 1010 | ≥ kd f |
| 9,10-dicyanoanthracene | −1.08 | −0.03 | −1.11 | 1.1 ±0.2 × 1010 | ≥ kd f |
| 9-cyanoanthracene | −0.56 | −0.04 | −0.60 | 6.7 ± 1.3 × 109 | 1.2 × 1010 |
| anthracene | −0.52 | −0.04 | −0.56 | 4.4 ± 0.4 × 109 | 6.0 × 109 |
| fluoranthene | −0.48 | −0.04 | −0.52 | 1.0 ± 0.1 × 109 | 1.1 × 109 |
| 9-phenylanthracene | −0.45 | −0.03 | −0.48 | 1.4 ± 0.2 × 109 | 1.5 × 109 |
| benzo[e]pyrene | −0.42 | −0.03 | −0.45 | 1.2 ± 0.1 × 109> | 1.3 × 109 |
| 9,10-diphenylanthracene | −0.39 | −0.03 | −0.42 | 1.3 ± 0.4 × 109 | 1.4 × 109 |
| 9-methylanthracene | −0.37 | −0.04 | −0.41 | 7.5 ± 0.4 × 108 | 7.9 × 108 |
| perylene | −0.32 | −0.04 | −0.36 | 5.8 ± 1.8 × 108> | 6.1 × 108 |
| benzo[c]phenanthrene | −0.29 | −0.04 | −0.33 | 6.3 ± 1.5 × 108 | 6.6 × 108 |
| benzo[a]pyrene | −0.14 | −0.03 | −0.17 | 5.5 ± 1.5 × 108 | 5.7 × 108 |
| [N(p-C6H4OMe)(p-C6H4Br)2]●+ | −0.04 | 0.00 | −0.04 | 5.3 ± 0.5 × 105 k | 5.3 × 105 |
| [N(tol)3]●+ h | 0.06 | 0.00 | 0.06 | 1.2 ±0.1 × 105 k | 1.2 × 105 |
| [N(p-C6H4OMe)3]●+ | 0.28 | 0.00 | 0.28 | 8 ± 2 × 102 k | 8 × 102 |
| 2,4,6-tBu3C6H2OH | ΔG°ET (eV) l | w (eV) b | ΔG°′ET (eV) c | kobs (M−1s−1) d | kCPET (s−1) e |
|---|---|---|---|---|---|
| [TPP](BF4) g | −0.95 | −0.03 | −0.98 | 1.5 ±0.1 × 1010 | ≥ kd f |
| 9,10-dicyanoanthracene | −0.34 | −0.03 | −0.37 | 8.6 ± 1.5 × 109 | ≥ kd f |
| 9-cyanoanthracene | 0.18 | −0.04 | 0.14 | 2.4 ± 0.5 × 109 | 2.8 × 109 |
| anthracene | 0.22 | −0.04 | 0.18 | ~6 × 107 m | 5.9 × 107 |
Calculated by the Rehm-Weller equation, equation 3, for photo-oxidations, using E(D+/D)CPET. These potentials therefore correspond to those of the CPET process.
From equation 4, using the equivalent radii of Tables S4 and S5.
From equation 4.75
Average and 1 standard deviation from three measurements with ~1–5% MeOH added by volume.
Calculated using equation 2, taking kD = 1010.2 M−1 s−1 and KA = 1 M−1; error bars are similar to those for kobs except are larger when kobs approaches kd.
Because kobs is very close to the diffusion limit, 1010.2 M−1 s−1, the data only show that kH,CPET ≥ kd.
2,4,6-triphenylpyrylium tetrafluoroborate
Tri-p-tolylaminium.
Methylphenothiazinium.
Values reported in reference 23.
Values reported in reference 21.
Calculated using eq 3 with E(D+/D)ET which is 0.6–0.8 eV more positive than E(D+/D)CPET for photo-oxidation involving the same polyarene.
Measuring this slow rate required extremely high phenol concentrations which could affect the characteristic radiative dynamics of the fluorophore, so the uncertainty on this value is larger than the ~2% estimated error from fitting the fluorescence data.
Fluorescence quenching must involve electron transfer (ET) rather than resonant energy transfer because the phenols are not significantly absorbing at the emission wavelengths of the fluorophores. The correlation of rate constants with excited state reduction potential of the fluorophores (Table 1 and Figure 2) also points to quenching by ET. However, simple ET without proton movement is ruled out by its much less favorable driving force.21,23,68 As shown in Table 1 below, rate constants of > 109 M−1 s−1 have been measured for reactions with CPET driving forces of −0.43 eV (HOAr-NH2), −0.03 eV (HOAr-Py), and −0.42 eV (HOAr-CH2Py). Such high rate constants would not be possible for ET processes that are 0.60–0.80 eV less favorable.
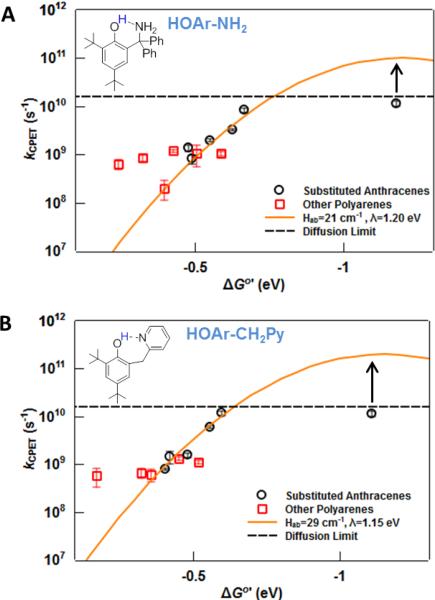
Figure 2.
Plots of photochemical kCPET values vs. ΔGo' for (A) HOAr-NH2 and (B) HOAr-CH2Py. The points for the reaction with TPP+ (the most exergonic of the photo-oxidations) occur at the diffusion limit and have been removed to allow for an expanded x-axis. The remaining points at the diffusion limit are indicated as lower limits. A curve that represents a good fit of eq 1 to the substituted anthracene data (black circles) is provided in each case.
The importance of proton transfer (PT) is also indicated by the substantially larger rate constants for the HOAr-B quenchers vs. 2,4,6-tBu3C6H2OH, which cannot undergo proton loss, with the same photo-oxidant. For example, with anthracene as the photo-oxidant, k > 109 M−1 s−1 for all three HOAr-B but k ≅ 6 × 107 M−1 s−1 for the same reaction with 2,4,6-tBu3C6H2OH, in the absence of an appended base. In a few cases, we observe kinetic isotope effects (KIEs) kH/kD > 1.5 (although the uncertainties in these measurements are high, see SI), which is also an indication of a CPET reaction. KIEs close to unity are still consistent with CPET, as has been discussed theoretically and seen experimentally.69 Finally, we note that mechanisms of initial pre-equilibrium PT followed by ET (termed PTET) were ruled out in earlier studies with these phenols, based on the very unfavorable initial PT step.23
Thus in essentially all of the cases, the fluorescence quenching is due to electron transfer from the phenol-base to the fluorophore concerted with intramolecular proton transfer from the phenol to the base. As noted above, such an MS-CPET mechanism has been shown to be followed by the phenols used here reacting with weaker oxidants,21,23,24,62 and, as shown below, there is good correspondence between the thermal and photo-induced rate constants. In general, oxidations of such H-bonded phenols have been shown to be CPET reactions,8–12,16–23,27,29,33,56,62,70,71 although Hammarström and coworkers have found exceptions.18,19
For reactions near the diffusion limit, diffusion and CPET are kinetically coupled to give the observed rate, kobs. Following the standard Marcus treatment of ET, diffusion forms a precursor complex with rate constant kd and equilibrium constant KA, and kCPET is taken as the first-order rate constant for CPET within this complex (Scheme 1A). For each of the phenols in this study at least several reactions occurred at the diffusion limit, as evidenced by a plateauing of kobs for the most exergonic reactions. kd was determined empirically to be 1010.2 M−1 s−1 from an average of these values. When kobs is close to kd, kCPET is calculated according to eq 2, with the standard assumption that KA ≅ 1 M−1.72 Uncertainties in the calculated values of kCPET that stem from the chosen value of KA and kd are discussed below.
| (2) |
The driving force for photo-induced electron transfer rate constants such as these is typically calculated by the method of Rehm and Weller (eq 3). This approach has been questioned by a recent re-examination of the original Rehm-Weller dataset. Farid et al. showed that the oxidations of substituted aryl compounds by excited state polyarenes can involve a significant role for fluorescent exciplexes, so that the typical Marcus formalism cannot be applied.73,74 For the reactions studied here, steady state fluorescence spectra indicate no fluorescing exciplexes across the entire span of driving forces, indicating that the Rehm-Weller equation is appropriate for calculating ΔGo for these reactions. However, in some instances of `slow' quenching, i.e. ΔGo > −0.1 eV and kobs < 108 M−1 s−1, the quenching observed by time-resolved fluorescence was not observed by steady-state fluorescence, and in some instances steady-state measurements showed an increase in fluorescence quantum yield in the presence of the phenol-base. This phenomenon can be accounted for by the large concentrations of phenol-base affecting the radiative and nonradiative deactivation pathways, leading to an increased fluorescence quantum yield.73 Thus these results do not represent true ET quenching of the polyarene fluorescence, and are not included in this report. Otherwise, the driving force can be calculated by eq 3 and the data reported here can be analyzed by the Stern-Volmer approach.
The Rehm-Weller equation (eq 3) gives the driving force for photo-induced electron transfer processes using the ground-state reduction potentials (E) and excited state energies (ΔG00). In this case, the measured reduction potential of the donor, E(D+/D), is for the CPET reaction, which includes the proton transfer.21,23E and ΔG00 values for all compounds are given in the SI.
| (3) |
| (4) |
The electron transfer step in this reaction converts a precursor complex to a successor complex, D|A → D+|A−. In this case, the successor complex is stabilized by the attraction of the opposite charges, and ΔGo must be corrected for this energy. The stabilization is −-e2/DrD+|A− (eq 4) for an excited neutral donor oxidizing a phenol, in which D is the static dielectric constant and rD+|A− is taken as the sum of the radii of the donor and acceptor. This term was calculated to be about −-30–40 meV for all neutral donor/acceptor combinations here. This correction is not needed for reactions of cationic acceptors with neutral donors, such as the photochemical reactions of the triphenylpyrylium cation and the stopped-flow reactions using triarylaminium radical cations. In this situation, the ET step is D|A+ → D+|A which occurs with very little change in the electrostatic interaction so w ≅ 0.
II. Analysis of the Rate Constants with the Semi-classical Marcus Equation
The rate constants measured here for photo-oxidation of HOAr-NH2 and HOAr-CH2Py are plotted as a function of ΔGo' in Figure 2. The estimated error bars are shown and are typically the size of the symbols or smaller. The rate constants for the reactions of substituted anthracenes are plotted with black circles, while the data for all of the other polyarenes are red squares. The rate constants for the substituted anthracenes follow the expected dependence of kCPET on ΔGo'. To highlight this dependence, Figure 2 includes parabolic fits that are derived from the combined datasets in Figure 3, as described below.
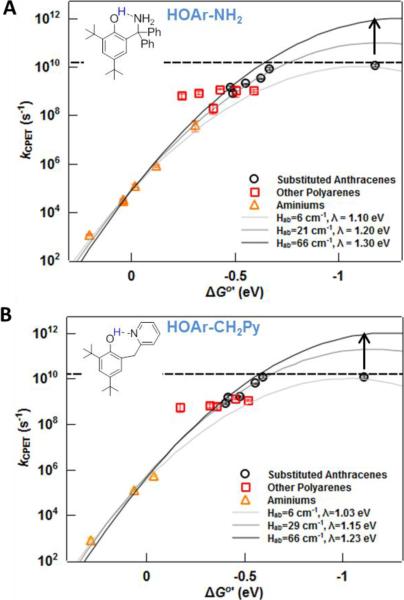
Figure 3.
kCPET plotted as a function of ΔGo' for (A) HOAr-NH2 and (B) HOAr-CH2Py for oxidation by both excited-state polyarenes (black circles and red squares) and aminiums (orange triangles). Note the log scale on the y-axis. Various Marcus parabolas with variable λ have been provided with pre-exponential terms spanning 1010–1012 s−1. The arrows in the plots indicate that these points, which were observed at the diffusion limit, are lower limits for kCPET. The other polyarenes (red squares) are not considered in making the fits.
The rate constants for the other polyarenes have a much flatter and more irregular dependence on ΔGo'. In the analyses below, we restrict our fitting to the data from the anthracene reactions, which follows most studies of photoinduced electron transfer reactions in which a series of structurally similar oxidants is typically used.11,12,73,74,76,77
The irregular dependence of rate constants for polyarenes other than the anthracenes indicates that there are differences between these photo-oxidants other than their excited state reduction potentials. Given their structural diversity, it is not surprising that reorganization energies and/or Hab values vary over this series. Consistent with this, the farthest outliers from the parabolic fits below are generally the larger polyarenes, for both the HOAr-NH2 and the HOAr-CH2Py reactions. To explore the origin of these irregularities, we have carried out independent calculations of the outer- and inner-sphere reorganization energies λo and λi. These are described in detail in the Supporting Information; only the approach and conclusions are summarized here. The λo and λi components are broken down into contributions from both the donor (the phenol) and acceptor (excited state arene). λo values for both the phenols and polyarenes were estimated from an empirical relation using equivalent hard-shell radii,55,78,79 and λi for the polyarenes was calculated using Nelsen's 4-point model.66,67 A formalism was developed for adjusting ΔGo' for differences in the calculated λ, which took into account the best fit parameters for the substituted anthracene curve for each phenol-base (see SI). The calculated values of λ for reactions involving the same phenol-base, which included both the phenol and photo-oxidant contributions, generally differed by no more than 0.1 eV. The ΔGo' datasets adjusted for these differences in λ still show significant outliers from the best fit. Thus differences in λ alone cannot account for the variation in kCPET.
This analysis indicates that the reaction pairs that are outliers have different adiabaticities (different values of Hab) from that of the best fit. The extent of the difference in adiabaticity was explored by maintaining the best-fit value of λ and optimizing Hab for the adjusted dataset to fit the Marcus parabola through the outlier points. This exercise indicated a substantially increased Hab term, relative to that for the substituted anthracenes, for these outlier points. It is less likely that these outliers are a result of different values of KA and kd for the larger polyarenes (vide infra and SI).
Figure 3 plots the photochemical rate constants together with the previously determined thermal rate constants for oxidations of HOAr-NH2 and HOAr-CH2Py with aminium ions from stopped-flow experiments (triangles).21,22,62 The inverted parabolas in the Figures are the predicted log(k) vs. ΔGo' curves from the semi-classical Marcus equation (eq 1), with the given values of Hab and the reorganization energy λ. The stopped-flow rate constants and the anthracene photochemical rate constants as a function of driving force are described with good accuracy by the same Marcus parabola. In fact, the best-fit parameters shown in Figure 2 are retained with the expanded dataset of Figure 3. The oxidations with aminium radical cations lie generally in the linear regime of the ET curve; therefore, many different electron transfer curves that represent large spans in Hab and λ can fit this data. That a single electron transfer curve can be employed to model the entire dataset is therefore not conclusive that the two sets of reactions share the same ET parameters. However, based on the mild curvature of the points for the aminium oxidations, the span of pre-exponential factors for equation 1 (“A”) can be narrowed to 1010 < A < 1012 s−1 for HOAr-NH2 and 108 < A < 1011 s−1 for HOAr-CH2Py, indicating similar Hab and λ values for both types of oxidations. This is consistent with reported values of λ for tri-p-tolylamine+/0 (0.51 eV in MeCN) and non-ion-paired anthracene0/− or tetracene0/− (both 0.38 eV in DMF).75 The apparent equivalence of the reorganization energies and Hab values for the anthracene photooxidations and the aminium thermal oxidations is reasonable given the similar size of these oxidants (both with three aromatic rings) and their somewhat localized charge (at the nitrogen in NAr3•+ and on the central ring in anthracene•−).
The combined stopped-flow and photochemical datasets are very well fit by the semi-classical Marcus equation (eq 1), over 107 in kCPET. This is a strong validation of the use of this equation to analyze CPET rate constants. Many years ago, Marcus predicted that this approach would hold for proton and atom transfers in this driving force regime, where −ΔGo < λ.80–82 The semi-classical Marcus equation is much simpler than current theories of CPET, particularly in the treatment of the pre-exponential factor. In Hammes-Schiffer's multistate continuum theory, this pre-exponential term is a sum of Boltzmann-weighted Franck-Condon overlaps of vibronic states integrated over a range of proton donor-acceptor distances. This detailed analysis is conceptually very important and is needed to understand detailed issues such as H/D kinetic isotope effects. However, at least for these two compounds, the rate constants over a span of nearly 0.9 eV in ΔGoCPET can be fit to good accuracy with a single Hab overlap parameter and a single intrinsic barrier λ. It is not evident from the more complete and complex theories that Hab should be essentially unchanged over such a range of reaction energies, especially given the importance of vibrational excited states in many cases.
The three parabolas drawn in Figure 3 have pre-exponential factors (“A”) that span from 1010–1012 s−1. For both phenol-bases, only electron transfer curves with A ≅ 1011 s−1 are successful in modeling the entire dataset. For reactions of HOAr-NH2, the parabola is defined by λ = 1.2 eV and A = 1011 s−1, which implies Hab = 21 cm−1. For HOAr-CH2Py, the derived values are similar: λ = 1.15 eV, A = 1011.3 s−1 and Hab = 29 cm−1. If the conservative estimate of ±20–30 meV is made for the uncertainty of each variable in equations 3 and 4, then the uncertainty in the corrected driving force ΔGo' is 30–40 meV, about the size of the points in Figures 2 and 3. Therefore, the Marcus parabolas in Figures 2 and 3 that fall on the points represent accurate estimates of the Marcus parameters; placing the fit outside of these points gives significantly different values of λ and/or Hab.
The situation is markedly different for the HOAr-Py system (Table 1, Fig S1). All of the photochemical rate constants kobs are ≥ 4 × 109 M−1 s−1, even at driving forces as low as ΔGo'= −0.03 eV. The kCPET values for reactions with substituted anthracenes are all ≥ kd. No thermal rate constants are available with aminium ions, because the reactions even at ΔGo'CPET = 0 are too fast to measure with stopped flow mixing.21,23 Therefore a detailed analysis similar to those above is not possible, and Hab or λ cannot be determined. Still, these data are consistent with the conclusion based on stopped flow measurements with iron-tris(diimine) oxidants that at ΔGo'CPET ≅ 0, HOAr-Py reacts roughly two orders of magnitude faster than HOAr-NH2 and HOAr-CH2Py.21,23 The kobs for the 9-phenylanthracene photo-oxidation of HOAr-Py reported here is almost at the diffusion limit, 1.1 ± 0.1 × 1010 M−1 s−1 at ΔGo' = −0.34 eV, while the values for the analogous oxidations of HOAr-NH2 and HOAr-CH2Py are almost an order of magnitude slower despite having larger driving forces: 1.8 ± 0.1 × 109 M−1 s−1 at ΔGo' = −0.55 eV and 1.4 ± 0.2 × 109 M−1 s−1 at ΔGo' = −0.48 eV, respectively. Consistent with these results, our computational estimate of λ for the reaction of HOAr-Py + excited anthracene (see Supplementary Information) is ~0.2 eV less than the corresponding values for either HOAr-NH2 or HOAr-CH2Py.
2,4,6-tBu3C6H2OH is 0.6–0.8 V more difficult to oxidize than the phenols with pendent bases.8,23 These reactions likely proceed by rate limiting ET followed by PT to the solvent or to the reduced arene. The 0.6–0.8 V difference in potential is the energetic benefit of transferring the proton concerted with electron transfer, the advantage of CPET over ET for the phenol-base compounds. The high potential for the oxidation of 2,4,6-tBu3C6H2OH limits the number of photochemical polyarene reactions that can be studied by the TCSPC method to only the most oxidizing photo-oxidants. Still, when ET from 2,4,6-tBu3C6H2OH has the same driving force as CPET from the phenol-bases, 2,4,6-tBu3C6H2OH is more reactive. For instance, 2,4,6-tBu3C6H2OH transfers an electron to the 9-cyanoanthracene excited state at 2.8 ± 0.5 × 109 M−1 s−1 despite that reaction being 0.14 eV uphill (Figure S1). This is consistent with prior studies showing that CPET is usually energetically favored over ET but is intrinsically more difficult.18,23,24,33,36 Note that the high rates of oxidation of 2,4,6-tBu3C6H2OH are achieved only with much stronger oxidants than used for the phenol-base compounds, because the ET reduction potential for 2,4,6-tBu3C6H2OH is much higher than the PCET reduction potential for HOAr-B. The data available for 2,4,6-tBu3C6H2OH do not indicate directly whether the slower rate constants for CPET vs. ET at the same driving force are due to CPET having a higher intrinsic barrier λ or being more non-adiabatic (lower Hab), although indirect assessments are possible, vide infra.
Uncertainties in the values of Hab for the HOAr-NH2 and HOAr-CH2Py datasets stem in part from uncertainties in the values of KA and kd, which affect kCPET via eq. 2). We have used the typical value of KA = 1 M−1; Sutin has argued that KA should have a value less than one while following Eberson an estimate of 2 M−1 is obtained.58,83,84 A change in KA by a factor f would change Hab by f−1/2, e.g. a 40% increase in Hab if KA is taken as 0.5 M−1. The value of kd (1010.2 M−1 s−1) was determined from the many measured rates near this value. If this value is in error, or is different for the different reagents, this would significantly affect kCPET for reactions with kobs near the diffusion limit. However, the value of kd does not affect kCPET when kobs is more than an order of magnitude smaller than kd. Choosing a value smaller than the apparent kd, such as 1010 s−1, changes the best-fit values only slightly because of the curvature of points with kobs ≤ 109 M−1 s−1 (Figure S2).
III. Thec λ and Hab values: Non-adiabaticity of the phenol oxidations
The dependence of the rate constants on ΔGo' (Figure 3) shows that CPET oxidations of HOAr-NH2 and HOAr-CH2Py by aminium ions or excited polyarenes are mildly non-adiabatic. The Hab values of 20–30 cm−1 are well below the 207 cm−1 (kBT) value for adiabatic processes. Stated another way, the pre-exponential factors from the fits above, log(A) = 11–11.3 for HOAr-NH2 and HOAr-CH2Py, are significantly lower than the Eyring pre-factor kBT/h in Transition State Theory, log(6 × 1012 s−1) = 12.8 at 298 K. These reactions, however, are not as non-adiabatic as ET between many transition metal systems, where Hab can be 1 cm−1 or less.61,85 Thus the non-adiabatic character of the HOAr-NH2 and HOAr-CH2Py reactions reduces their rate constants by factors of ca. 60 and 30, respectively, versus what would be predicted for an adiabatic reaction. In simple terms, the developers of CPET theories are correct that non-adiabatic effects are both conceptually and quantitatively important. On the other hand, this slowing of the rate constant by a factor of ~45 is within the uncertainty of ab initio or DFT calculations for solution reactions of this complexity86 (a decrease by a factor of 40 in k results from an increase in ΔG‡ of 2.2 kcal mol−1). Thus it is reasonable for computational chemists to neglect non-adiabatic effects when calculating rate constants and energy surfaces for PCET reactions of this kind.
Several reports discuss the extent of non-adiabaticity in this family of reactions. These studies used various assumptions to simplify eq 1 to allow extraction of λ and/or Hab. Our laboratory used a variable temperature stopped-flow study, of oxidations of HOAr-NH2 by aminiums and HOAr-Py by tris-diimine ferric complexes, to indicate lower limits for Hab of ca. 10 and 6 cm−1.23 Costentin et al. re-evaluated these data using new electrochemical results and a different version of eq 1, finding λ = 0.8 eV for HOAr-NH2 and that the reactions are non-adiabatic with a transmission coefficient on the order of 0.005 (Hab ~ 14 cm−1).27 For the closely related aminophenol HOAr-CH2NC4H8, these researchers estimated λ = 0.7 eV based on electrochemical measurements,20 then later reported λ = 1.06 eV for this aminophenol and that this electrochemical reaction is adiabatic.27,41 A later analysis that took into account the distance dependence of the reaction revealed λ = 1.4 eV for HOAr-CH2NC4H8 and λ = 1.5 eV for HOAr-NH2 and mild non-adiabaticity for these reactions.87,88 Their analysis also indicated that excited proton vibrational states play a small role in the reaction. In contrast, Thorp and Meyer's study of intermolecularly H-bonded tyro-sines with Ru or Os photo-oxidants found that kCPET varied monotonically with ΔGoCPET, but that a single-mode model was insufficient to explain the data and therefore concluded that vibronic levels above υ = 0 participate in the CPET process.49 The Hab term has been explored by Hammarström and coworkers for similar photo-oxidations by Ru complexes. A Ru-tyrosine conjugate was concluded to have Hab = 5 cm−1 and λ = 1.2 eV for pure electron transfer, versus Hab = 7 cm−1 and λ = 2.4 eV for the related CPET reaction (as determined via variable pH and temperature data; this large value of λ was determined without taking the increase in proton entropy with temperature into account).33 In a different report the oxidation of a phenolcarboxylate intramolecular species by excited Ru(bpy)32+ was explored; λ = 0.9–1.2 eV was determined, and no value for Hab was given.19 Overall, it appears that the non-electrochemical determinations of λ and Hab for HOAr-NH2 and similar systems roughly agree with the values determined in this report, with the exception of the value of λ determined in reference27. In all cases, save the earlier reports of electrochemical oxidations of aminophenols, these reactions were concluded to be mildly non-adiabatic.
The method used in this paper presents the most direct method for measuring λ and Hab, i.e., by monitoring the rate of the reaction as a function of driving force and fitting the data to eq 1. The collection of data close to the top of the Marcus parabola, as presented herein, is essential to accurate elucidation of these parameters. Future studies in our laboratory will extend these studies to unimolecular reactions that are not limited by diffusion and will therefore be able to probe closer to the top of the parabola.
Theoretical studies suggest that the degree of electron-proton non-adiabaticity is related to the extent of charge distribution in a PCET process, with large redistribution of charge corresponding to a non-adiabatic process.1,6,89,90 This predicts that the limiting examples of hydrogen atom transfer (HAT), in which the e− and the H+ transfer together from a single location to another, will be an adiabatic process. In contrast, multiple-site concerted proton-electron transfer (MS-CPET) processes should be non-adiabatic, with maximum rate constants slower than those for HAT.1,89,91 The results reported here provide support for the latter prediction. The former prediction is also supported by previous work from our lab on hydrogen atom transfer reactions. For a large set of HAT reactions, the classical (adiabatic) version of Marcus theory holds well in most cases.28,50,51,54
The intrinsic barriers of 1.20 and 1.15 eV for HOAr-NH2 and HOAr-CH2Py are larger than typical λ values for simple organic ET reactions. For instance, ET self-exchange of the aminium ions used in the stopped flow measurements has λ = 0.5 eV (assuming an adiabatic reaction).92 Similarly, the data suggest that ET from 2,4,6-tBu3C6H2OH is intrinsically easier than CPET of HOAr-NH2 and HOAr-CH2Py. The outer-sphere (solvent) reorganization energies are likely not so different for ET and CPET for these molecules, as the proton moves only a short distance (~0.7 Å) within the phenol-base.22 The higher values of λ for HOAr-NH2 and HOAr-CH2Py are therefore likely due to higher inner-sphere reorganization energies, particularly distortions that reduce the proton donor-acceptor distance and thereby facilitate CPET.22 Still, the λ values of slightly more than 1 eV are in a range that is reasonable for biological reactions, consistent with the involvement of tyrosine-base ET cofactors.10,60,93
The CPET reactions of HOAr-NH2 and HOAr-CH2Py are very similar in their λ and Hab parameters, while the reactions of HOAr-Py are much more facile. The total reorganization energy for the reactions of HOAr-Py, HOAr-NH2, and HOAr-CH2Py with anthracene can be calculated using Nelsen's 4-point model66 for λi contributions and an empirical method for calculating the λo contributions.21,62,94 These calculations indicate that λ for the reaction of HOAr-Py and anthracene is ~0.2 eV smaller than the calculated values for the reactions of the unconjugated phenol-bases. The much faster reactions of HOAr-Py, despite the fact that pyridine is a weaker base than the primary amine, were ascribed to conjugation of the pyridine with the phenol and the resulting resonance-assisted hydrogen bond.21 For the reaction of HOAr-Py with excited substituted anthracenes, the kCPET rate constants being at least 1010 s−1 and the calculated value of λCPET ≅ 1 eV imply that A ≥ 1012 s−1 and Hab ≥ 60 cm−1. These values provide the Marcus parabola with the smallest pre-exponential factor that can account for all of the reactions of the substituted anthracenes having kCPET ≥ kd. These calculations and estimates indicate that the more facile reactions of HOAr-Py stem from not only a smaller inner-sphere reorganization energy, but also from greater electronic coupling.
The Marcus equation (eq 1) famously predicts an inverted region for ET, where the rate constants decrease with more negative driving forces (−ΔGo' > λ). In systems examined here, the reactions of the excited state triphenylpyrylium cation are predicted to be in the inverted region (Figure 2 and Table 1), but they all occur at the diffusion limit. No evidence of inverted behavior is seen. Even for ET, the lack of inverted region is a general result for bimolecular reactions75,85 (with a few exceptions, cf.,61,85,95,96). This has been attributed to charge transfer occurring at increasing distances in solution as the driving force is increased, which changes the reorganization energy.75 Recent theoretical work concluded that the inverted region is very likely inaccessible for CPET (unless the proton transfer distance increases with driving force).82 In addition, the triphenylpyrylium ion excited state is so oxidizing that the observed quenching could occur by simple ET, without proton movement, and still occur at the diffusion limit. While the lack of observation of an inverted region for CPET is not surprising, this is one of the rare experimental tests in that region.
Conclusions
A central question in fundamental studies of multiple-site concerted proton-electron transfer (MS-CPET) is whether these reactions should be considered adiabatic or non-adiabatic. In this study of hydrogen-bonded phenol-base compounds, the oxidations by photoexcited anthracenes and by aminium radical cations are shown to be non-adiabatic by direct experimental measurements. The rate constants for two different phenols, over a range of > 107 in kCPET and almost 0.9 eV in driving force (ΔGo'), are well described by semi-classical Marcus theory. For HOAr-NH2 and for HOAr-CH2Py, each set of data is well fit by a single Marcus parabola, with λCPET ≅ 1.15–1.20 eV and Hab ≅ 20–30 cm−1. It is significant that for each of the phenols, the whole dataset can be fit with a single intrinsic barrier and vibronic coupling parameter Hab. For the phenol-pyridine compound HOAr-Py, the fast rate constants appear to be due to both a smaller reorganization energy and a larger Hab, both likely resulting from the conjugation between the phenol and the base. For all three phenol-bases the Hab values are indicated to vary significantly for structurally different polyarene photo-oxidants.
The non-adiabatic character of the reactions reduces their rate constants by about a factor of ca. 45 versus an adiabatic reaction with the same free-energy barrier. Thus CPET theories that emphasize the importance of non-adiabaticity are correct that this is a significant effect for the experimentally-measured kinetics of these reactions. On the other hand, computational studies are not introducing substantial error by ignoring non-adiabaticity because the factor of ~45 in rate constant is within the uncertainty of the calculations for such complex solution-phase reactions. That semi-classical Marcus theory can model the rates of these reactions over 7 orders of magnitude implies that complicated theoretical approaches are not necessarily required in the modeling of rate constants for this family of reactions.
Supplementary Material
ACKNOWLEDGMENT
We are grateful for financial support from the U.S. National Institutes of Health (GM-50422 and an ARRA collaborative supplement to that award). The UW Chemistry computing facilities have been generously supported by the University of Washington Student Technology Fee Program.
Footnotes
Supporting Information. Materials and methods, additional figures, and a calculations of reorganization energies. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- (1).Hammes-Schiffer S, Stuchebrukhov AA. Chem. Rev. 2010;110:6939. doi: 10.1021/cr1001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Cukier RI. Biochim. Biophys. Acta-Bioenerg. 2004;1655:37. doi: 10.1016/j.bbabio.2003.06.011. [DOI] [PubMed] [Google Scholar]
- (3).Cukier RI, Nocera DG. Annu. Rev. Phys. Chem. 1998;49:337. doi: 10.1146/annurev.physchem.49.1.337. [DOI] [PubMed] [Google Scholar]
- (4).Iyengar SS, Sumner I, Jakowski J. J. Phys. Chem. B. 2008;112:7601. doi: 10.1021/jp7103215. [DOI] [PubMed] [Google Scholar]
- (5).Siegbahn PEM, Blomberg MRA. Chem. Rev. 2010;110:7040. doi: 10.1021/cr100070p. [DOI] [PubMed] [Google Scholar]
- (6).Hammes-Schiffer S. J. Phys. Chem. Lett. 2011;2:1410. doi: 10.1021/jz101532g. [DOI] [PubMed] [Google Scholar]
- (7).Hammes-Schiffer S. Energ. Environ. Sci. 2012;5:7696. [Google Scholar]
- (8).Warren JJ, Tronic TA, Mayer JM. Chem. Rev. 2010;110:6961. doi: 10.1021/cr100085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Meyer TJ, Huynh MHV, Thorp HH. Angew. Chem. Int. Ed. 2007;46:5284. doi: 10.1002/anie.200600917. [DOI] [PubMed] [Google Scholar]
- (10).Dempsey JL, Winkler JR, Gray HB. Chem. Rev. 2010;110:7024. doi: 10.1021/cr100182b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Biczók L, Gupta N, Linschitz H. J. Am. Chem. Soc. 1997;119:12601. [Google Scholar]
- (12).Biczok L, Linschitz H. J. Phys. Chem. 1995;99:1843. [Google Scholar]
- (13).Shukla D, Young RH, Farid S. J. Phys. Chem. A. 2004;108:10386. [Google Scholar]
- (14).Concepcion JJ, Brennaman MK, Deyton JR, Lebedeva NV, Forbes MDE, Papanikolas JM, Meyer TJ. J. Am. Chem. Soc. 2007;129:6968. doi: 10.1021/ja069049g. [DOI] [PubMed] [Google Scholar]
- (15).Bonin J, Costentin C, Louault C, Robert M, Savéant J-M. J. Am. Chem. Soc. 2011;133:6668. doi: 10.1021/ja110935c. [DOI] [PubMed] [Google Scholar]
- (16).Bonin J, Costentin C, Robert M, Saveant J-M. Org. Biomol. Chem. 2011;9:4064. doi: 10.1039/c1ob05090g. [DOI] [PubMed] [Google Scholar]
- (17).Bonin J, Costentin C, Robert M, Savéant J-M, Tard C. Acc. Chem. Res. 2011 doi: 10.1021/ar200132f. [DOI] [PubMed] [Google Scholar]
- (18).Sjodin M, Ghanem R, Polivka T, Pan J, Styring S, Sun L, Sundstrom V, Hammarstrom L. Phys. Chem. Chem. Phys. 2004;6:4851. [Google Scholar]
- (19).Sjödin M, Irebo T, Utas JE, Lind J, Merényi G, Åkermark B, Hammarström L. J. Am. Chem. Soc. 2006;128:13076. doi: 10.1021/ja063264f. [DOI] [PubMed] [Google Scholar]
- (20).Costentin C, Robert M, Savéant J-M. J. Am. Chem. Soc. 2006;128:4552. doi: 10.1021/ja060527x. [DOI] [PubMed] [Google Scholar]
- (21).Markle TF, Mayer JM. Angew. Chem. Int. Ed. 2008;47:738. doi: 10.1002/anie.200702486. [DOI] [PubMed] [Google Scholar]
- (22).Markle TF, Rhile IJ, Mayer JM. J. Am. Chem. Soc. 2011;133:17341. doi: 10.1021/ja2056853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Rhile IJ, Markle TF, Nagao H, DiPasquale AG, Lam OP, Lockwood MA, Rotter K, Mayer JM. J. Am. Chem. Soc. 2006;128:6075. doi: 10.1021/ja054167+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rhile IJ, Mayer JM. J. Am. Chem. Soc. 2004;126:12718. doi: 10.1021/ja031583q. [DOI] [PubMed] [Google Scholar]
- (25).Maki T, Araki Y, Ishida Y, Onomura O, Matsumura Y. J. Am. Chem. Soc. 2001;123:3371. doi: 10.1021/ja002453+. [DOI] [PubMed] [Google Scholar]
- (26).Benisvy L, Blake AJ, Collison D, Stephen Davies E, David Garner C, McInnes EJL, McMaster J, Whittaker G, Wilson C. Dalton T. 2003;258 doi: 10.1039/b513221p. [DOI] [PubMed] [Google Scholar]
- (27).Costentin C, Robert M, Savéant J-M. J. Am. Chem. Soc. 2007;129:9953. doi: 10.1021/ja071150d. [DOI] [PubMed] [Google Scholar]
- (28).Mayer JM. Acc. Chem. Res. 2011;44:36. doi: 10.1021/ar100093z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Markle TF, Rhile IJ, DiPasquale AG, Mayer JM. Proc. Natl. Acad. Sci. USA. 2008;105:8185. doi: 10.1073/pnas.0708967105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Bronner C, Wenger OS. J. Phys. Chem. Lett. 2011;3:70. [Google Scholar]
- (31).Johannissen LO, Irebo T, Sjödin M, Johansson O, Hammarström L. J. Phys. Chem. B. 2009;113:16214. doi: 10.1021/jp9048633. [DOI] [PubMed] [Google Scholar]
- (32).Zhang MT, Irebo T, Johansson O, Hammarstrom L. J. Am. Chem. Soc. 2011;133:13224. doi: 10.1021/ja203483j. [DOI] [PubMed] [Google Scholar]
- (33).Sjödin M, Styring S, Wolpher H, Xu Y, Sun L, Hammarström L. J. Am. Chem. Soc. 2005;127:3855. doi: 10.1021/ja044395o. [DOI] [PubMed] [Google Scholar]
- (34).Reece SY, Nocera DG. J. Am. Chem. Soc. 2005;127:9448. doi: 10.1021/ja0510360. [DOI] [PubMed] [Google Scholar]
- (35).Magnuson A, Berglund H, Korall P, Hammarstrom L, Akermark B, Styring S, Sun LC. J. Am. Chem. Soc. 1997;119:10720. [Google Scholar]
- (36).Sjodin M, Styring S, Akermark B, Sun LC, Hammarstrom L. J. Am. Chem. Soc. 2000;122:3932. [Google Scholar]
- (37).Westlake BC, Brennaman MK, Concepcion JJ, Paul JJ, Bettis SE, Hampton SD, Miller SA, Lebedeva NV, Forbes MDE, Moran AM, Meyer TJ, Papanikolas JM. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8554. doi: 10.1073/pnas.1104811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Irebo T, Reece SY, Sjodin M, Nocera DG, Hammarstrom L. J. Am. Chem. Soc. 2007;129:15462. doi: 10.1021/ja073012u. [DOI] [PubMed] [Google Scholar]
- (39).Lachaud F, Quaranta A, Pellegrin Y, Dorlet P, Charlot M-F, Un S, Leibl W, Aukauloo A. Angew. Chem. Int. Ed. 2005;44:1536. doi: 10.1002/anie.200461948. [DOI] [PubMed] [Google Scholar]
- (40).Moore GF, Hambourger M, Gervaldo M, Poluektov OG, Rajh T, Gust D, Moore TA, Moore AL. J. Am. Chem. Soc. 2008;130:10466. doi: 10.1021/ja803015m. [DOI] [PubMed] [Google Scholar]
- (41).Costentin C, Robert M, Savéant J-M, Tard C. Angew. Chem. Int. Ed. 2010;49:3803. doi: 10.1002/anie.200907192. [DOI] [PubMed] [Google Scholar]
- (42).Osako T, Ohkubo K, Taki M, Tachi Y, Fukuzumi S, Itoh S. J. Am. Chem. Soc. 2003;125:11027. doi: 10.1021/ja029380+. [DOI] [PubMed] [Google Scholar]
- (43).Markle TF, Tenderholt AL, Mayer JM. J. Phys. Chem. B. 2012;116:571. doi: 10.1021/jp2091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Carra C, Iordanova N, Hammes-Schiffer S. J. Phys. Chem. B. 2002;106:8415. [Google Scholar]
- (45).Hatcher E, Soudackov AV, Hammes-Schiffer S. J. Am. Chem. Soc. 2004;126:5763. doi: 10.1021/ja039606o. [DOI] [PubMed] [Google Scholar]
- (46).Iordanova N, Decornez H, Hammes-Schiffer S. J. Am. Chem. Soc. 2001;123:3723. doi: 10.1021/ja0100524. [DOI] [PubMed] [Google Scholar]
- (47).Ishikita H, Soudackov AV, Hammes-Schiffer S. J. Am. Chem. Soc. 2007;129:11146. doi: 10.1021/ja072708k. [DOI] [PubMed] [Google Scholar]
- (48).Soudackov AV, Hazra A, Hammes-Schiffer S. J. Chem. Phys. 2011;135 doi: 10.1063/1.3651083. [DOI] [PubMed] [Google Scholar]
- (49).Fecenko CJ, Thorp HH, Meyer TJ. J. Am. Chem. Soc. 2007;129:15098. doi: 10.1021/ja072558d. [DOI] [PubMed] [Google Scholar]
- (50).Mayer JM. J. Phys. Chem. Lett. 2011;2:1481. doi: 10.1021/jz200021y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Warren JJ, Mayer JM. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5282. doi: 10.1073/pnas.0910347107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zieba AA, Richardson C, Lucero C, Dieng SD, Gindt YM, Schelvis JPM. J. Am. Chem. Soc. 2011;133:7824. doi: 10.1021/ja2001488. [DOI] [PubMed] [Google Scholar]
- (53).Yoder JC, Roth JP, Gussenhoven EM, Larsen AS, Mayer JM. J. Am. Chem. Soc. 2003;125:2629. doi: 10.1021/ja0273905. [DOI] [PubMed] [Google Scholar]
- (54).Mayer JM. Annu. Rev. Phys. Chem. 2004;55:363. doi: 10.1146/annurev.physchem.55.091602.094446. [DOI] [PubMed] [Google Scholar]
- (55).Saveant J-M. Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry. John Wiley & Sons, Inc.; Hoboken, New Jersey: 2006. pp. 59–60. [Google Scholar]
- (56).Bonin J, Costentin C, Louault C, Robert M, Routier M, Savéant J-M. Proc. Natl. Acad. Sci. USA. 2010;107:3367. doi: 10.1073/pnas.0914693107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Costentin C. Chem. Rev. 2008;108:2145. doi: 10.1021/cr068065t. [DOI] [PubMed] [Google Scholar]
- (58).Newton MD, Sutin N. Ann. Rev. Phys. Chem. 1984;35:437. [Google Scholar]
- (59).Winkler JR, Gray HB. Chem. Rev. 1992;92:369. [Google Scholar]
- (60).Gray HB, Winkler JR. Q. Rev. Biophys. 2003;36:341. doi: 10.1017/s0033583503003913. [DOI] [PubMed] [Google Scholar]
- (61).Turró C, Zaleski JM, Karabatsos YM, Nocera DG. J. Am. Chem. Soc. 1996;118:6060. [Google Scholar]
- (62).Markle TF. PhD Dissertation. University of Washington; 2009. [Google Scholar]
- (63).Inoue Y, Nakano T, Tanaka H, Kashiwa N, Fujita T. Chem. Lett. 2001;30:1060. [Google Scholar]
- (64).FluoFit Version 3.3. by PicoQuant GmbH. http://www.picoquant.com/
- (65).Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, J. A. Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian, Inc.; Wallingford CT: 2009. [Google Scholar]
- (66).Nelsen SF, Blackstock SC, Kim Y. J. Am. Chem. Soc. 1987;109:677. [Google Scholar]
- (67).Nelsen SF, Weaver MN, Luo Y, Pladziewicz JR, Ausman LK, Jentzsch TL, O'Konek JJ. J. Phys. Chem. A. 2006;110:11665. doi: 10.1021/jp064406v. [DOI] [PubMed] [Google Scholar]
- (68).This number represents an approximate average for the three phenol-base compounds as compared to the free 2,4,6-tri-tertbutylphenol. See Table S1 for the oxidation potentials of these four compounds.
- (69).Linschitz and coworkers report that the CPET oxidation of pmethoxyphenol/pyridine by singlet tetracene shows a significant KIE, whereas the much faster reaction with triplet fullerene has no KIE (Biczok, L.; Linschitz, H. J. Phys. Chem. 1995, 99, 1843) In a later report, they report kH/kD ratios significantly greater than 1 for phenol/pyridine systems in benzonitrile, but no KIE when the added base is DMSO (Biczók, L.; Gupta, N.; Linschitz, H. J. Am. Chem. Soc. 1997, 119, 12601). In general, KIE's greater than ~ 6 are indicative of vibronic or vibrational nonadiabaticity, but a small KIE does not rule out a nonadiabatic CPET event because of confounding effects from proton donor-acceptor motion and contributions from excited vibronic states (see: Hammes-Schiffer, S.; Soudackov, A. V. J. Phys. Chem. B 2008, 112, 14108. Edwards, S. J.; Soudackov, A. V.; Hammes-Schiffer, S. J. Phys. Chem. A 2009, 113, 2117). Therefore, excited vibronic contributions are a plausible factor that can account for the small KIEs if the promoting modes in these systems are low-energy vibrations. As reported previously, a significant KIE is observed for all oxidations by aminium oxidants (see: Markle, T. F.; Mayer, J. M. Angew. Chem. Int. Ed. 2008, 47, 738. Rhile, I. J.; Markle, T. F.; Nagao, H.; DiPasquale, A. G.; Lam, O. P.; Lockwood, M. A.; Rotter, K.; Mayer, J. M., J. Am. Chem. Soc. 2006, 128, 6075. Markle, T. F. PhD Dissertation, University of Washington, 2009).
- (70).Fecenko CJ, Meyer TJ, Thorp HH. J. Am. Chem. Soc. 2006;128:11020. doi: 10.1021/ja061931z. [DOI] [PubMed] [Google Scholar]
- (71).Huynh MHV, Meyer TJ. Chem. Rev. 2007;107:5004. doi: 10.1021/cr0500030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Eberson L. Adv. Phys. Org. Chem. 1982;18:79. [Google Scholar]
- (73).Farid S, Dinnocenzo JP, Merkel PB, Young RH, Shukla D, Guirado G. J. Am. Chem. Soc. 2011;133:11580. doi: 10.1021/ja2024367. [DOI] [PubMed] [Google Scholar]
- (74).Farid S, Dinnocenzo JP, Merkel PB, Young RH, Shukla D. J. Am. Chem. Soc. 2011;133:4791. doi: 10.1021/ja104536j. [DOI] [PubMed] [Google Scholar]
- (75).Eberson L. Electron Transfer Reactions in Organic Chemistry. Vol. 25. Springer-Verlag; Berlin: 1987. pp. 27–35. [Google Scholar]
- (76).Amada I, Yamaji M, Sase M, Shizuka H. J. Chem. Soc. Faraday Trans. 1995;91:2751. [Google Scholar]
- (77).Anbazhagan V, Kathiravan A, Asha Jhonsi M, Renganathan R. Z. Phys. Chem. 2007;221:929. [Google Scholar]
- (78).Kojima H, Bard AJ. J. Am. Chem. Soc. 1975;97:6317. [Google Scholar]
- (79).Peover ME. Oxidation and Reduction of Aromatic Hydrocarbon Molecules at Electrodes. Wiley; New York: 1971. pp. 259–281. [Google Scholar]
- (80).Marcus RA. J. Phys. Chem. 1968;72:891. [Google Scholar]
- (81).Marcus RA. J. Phys. Chem. B. 2007;111:6643. doi: 10.1021/jp071589s. [DOI] [PubMed] [Google Scholar]
- (82).Edwards SJ, Soudackov AV, Hammes-Schiffer S. J. Phys. Chem. B. 2009;113:14545. doi: 10.1021/jp907808t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Sutin N. Prog. Inorg. Chem. 1984;30:441. [Google Scholar]
- (84).Sutin's lower estimate derives from an analysis based on electron transfer occurring at a distribution of distances. For uncharged reactants, Eberson estimates KA to be 0.32, 0.86 and 1.84 M-1 for reaction distances of 5, 7 and 9 Å (Eberson, L., Electron-Transfer Reactions in Organic Chemistry. Advances in Physical Organic Chemistry 1982, 18, 79-185). Using these approximate values and the calculated separations of 9–13 Å (Table S5), an estimate of KA ~ 2 M-1 is made, leading to values of Hab that are more non-adiabatic than the values presented above.
- (85).Meyer TJ, Taube H. Electron transfer reactions. Vol. 1. Pergamon; New York: 1987. pp. 331–384. [Google Scholar]
- (86).Zhao Y, González-García N, Truhlar DG. J. Phys. Chem. A. 2005;109:2012. doi: 10.1021/jp045141s. [DOI] [PubMed] [Google Scholar]
- (87).Saveant J-M. Energy. Environ. Sci. 2012;5:7718. doi: 10.1039/C4EE01709A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Costentin C, Robert M, Saveant J-M. Phys. Chem. Chem. Phys. 2010;12:11179. doi: 10.1039/c0cp00063a. [DOI] [PubMed] [Google Scholar]
- (89).Sirjoosingh A, Hammes-Schiffer S. J. Phys. Chem. A. 2011;115:2367. doi: 10.1021/jp111210c. [DOI] [PubMed] [Google Scholar]
- (90).Georgievskii Y, Stuchebrukhov AA. J. Chem. Phys. 2000;113:10438. doi: 10.1063/1.1796751. [DOI] [PubMed] [Google Scholar]
- (91).Skone JH, Soudackov AV, Hammes-Schiffer S. J. Am. Chem. Soc. 2006;128:16655. doi: 10.1021/ja0656548. [DOI] [PubMed] [Google Scholar]
- (92).Sorensen SP, Bruning WH. J. Am. Chem. Soc. 1973;95:2445–2451. assuming an adiabatic reaction (λ = 4ΔG‡self-exchange) [Google Scholar]
- (93).Moser CC, Anderson JLR, Dutton PL. BBA-Bioenergetics. 2010;1797:1573. doi: 10.1016/j.bbabio.2010.04.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).The 4-point calculation predicts a ~ 0.4 eV smaller innersphere reorganization energy for HOAr-Py as compared to the unconjugated phenol-bases. The difference of ~0.2 eV in the total reorganization energy for the reaction with anthracene is obtained when taking into account the ~=0.2 eV calculated innersphere reorganization energy for the polyarenes via the additivity postulate, and assuming λo = 0.5 eV for the outersphere reorganization energy of the phenols. The outersphere contributions for the polyarenes is calculated by equation S10. All of these contributions are combined using equations S5 and S6 to calculate the total reorganization energy for the reaction.
- (95).McCleskey TM, Winkler JR, Gray HB. J. Am. Chem. Soc. 1992;114:6935. [Google Scholar]
- (96).Gould IR, Farid S. Acc. Chem. Res. 1996;29:522. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.