Abstract
The delta opioid receptor agonist SNC80 produces both antinociceptive and antidepressant effects in rodents. This profile suggests that SNC80 may also reverse prodepressant effects of pain. Accordingly, this study compared SNC80 effects in complementary assays of pain-stimulated and pain-depressed behavior in rats. Intraperitoneal injection of dilute acid served as an acute noxious visceral stimulus in rats to stimulate abdominal stretching (a pain-stimulated behavior) or depress intracranial self-stimulation of the medial forebrain bundle (ICSS; a pain-depressed behavior). When administered once per week to minimize acute tolerance, SNC80 (1-10 mg/kg IP) decreased acid-stimulated stretching but had little effect on acid-induced depression of ICSS. More frequent SNC80 administration produced tolerance to SNC80 effects on acid-stimulated stretching, but unmasked antinociception in the assay of acid-depressed ICSS. SNC80 did not facilitate ICSS in the absence of pain, and effects of SNC80 were not duplicated by ARM390, a delta agonist congener of SNC80 that does not internalize delta receptors. These findings support continued consideration of delta agonists as candidate analgesics to treat prodepressant effects of pain and illustrate the potential for diametrically opposite effects of drug treatments on preclinical measures of pain-stimulated and pain-depressed behavior.
Perspective
The delta opioid agonist SNC80 blocked pain-related depression of intracranial self-stimulation in rats, suggesting that delta agonists may be useful to treat prodepressant effects of pain. Repeated SNC80 produced tolerance to SNC80 antinociception in a conventional assay of pain-stimulated behavior but unmasked SNC80 antinociception in an assay of pain-depressed behavior.
Keywords: Pain, depression, delta opioid receptor, SNC80, ARM390
INTRODUCTION
Pain and depression are significant public health problems that often coexist 2,18,19,24, and the high comorbidity between pain and depression has been one factor contributing to increased preclinical investigation and clinical use of monoamine-uptake-inhibitor antidepressants for the treatment of pain 13,25. Delta opioid receptor agonists constitute another class of drugs that produce both antidepressant and antinociceptive effects in preclinical assays 20,34. In rodents, for example, the selective and high-efficacy delta agonist SNC80 produces an antidepressant-like reduction in immobility in the forced swim test 8,21 as well as blockade of nociceptive responses elicited by chemical irritants 16,42 and nociceptive hypersensitivity in models of inflammatory pain 5,15-17. These results suggest that delta agonists may have therapeutic utility for the treatment of pain and pain-related depression.
In view of this antinociceptive/antidepressant profile of delta agonists such as SNC80, the purpose of the present study was to compare effects of SNC80 in complementary assays of pain-stimulated and pain-depressed behavior in rats 29,31-33,36. Most preclinical assays of nociception measure pain-stimulated behaviors, which can be defined as behaviors (e.g. withdrawal responses) that increase in rate or intensity in the presence of a putative pain stimulus. However, pain is also associated with the depression of many behaviors (e.g. feeding, locomotion and positively reinforced operant behavior), and more generally, pain-depressed behaviors can be defined as behaviors that decrease in rate or intensity in the presence of a pain stimulus. Pain-depressed behaviors are commonly assessed in veterinary and human medicine to diagnose the presence of pain and the efficacy of treatments 10,11,14, and preclinical research has begun to develop and deploy assays of pain-depressed behavior to complement and extend existing assays of pain-stimulated behavior 27-29,33. In the present study, intraperitoneal injection of dilute lactic acid served as an acute noxious visceral stimulus to stimulate an abdominal stretching response and to depress intracranial self-stimulation of the medial forebrain bundle (ICSS) in rats. ICSS was used as the behavioral baseline for studies of pain-depressed behavior because (a) its stability over time renders it sensitive to changes produced by pain and drug manipulations, and (b) operant responding is maintained by direct stimulation of excitatory inputs to the mesolimbic dopamine system, a neural circuit implicated in the expression of motivated behavior and likely to be involved in mediating the prodepressant effects of pain 4,9,29,39.
Three sets of experiments were conducted. First, effects of acute SNC80 were examined on both acid-stimulated stretching and acid-depressed ICSS. We hypothesized that SNC80 would produce dose-dependent antinociception in both assays. Second, previous studies have shown that repeated treatment with SNC80 or related delta agonists produces rapid tolerance to antinociception in conventional assays of pain-stimulated behavior and to some other effects without producing tolerance to antidepressant effects 6,7,22,38. Accordingly, we examined effects of repeated SNC80 and hypothesized that tolerance would develop to antinociceptive effects in the assay of acid-stimulated stretching but not in the assay of acid-depressed ICSS. Lastly, we examined effects of ARM390, a congener of SNC80 reported to have similar potency and efficacy to SNC80 as a delta receptor agonist but a lower propensity to promote receptor internalization and produce acute antinociceptive tolerance 26,37,38. We hypothesized that ARM390 would produce antinociception in assays of both acid-stimulated stretching and acid-depressed ICSS after acute treatment and sustained antinociception after repeated treatment in both procedures.
METHODS
Subjects
Male Sprague-Dawley rats (Harlan, Frederick MD) with initial weights of 295-335 g were used for the studies of ICSS and lactic acid-induced stretching. Rats were individually housed and maintained on a 12-h light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. Food and water were continuously available except during experimental sessions. Animal maintenance and research were in compliance with NIH guidelines on care and use of animal subjects in research, and all animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
All animals were anesthetized with isoflurane gas (2.5-3% in oxygen; Webster Veterinary, Phoenix, AZ) for stereotaxic surgery lasting approximately 30 min to implant a stainless steel bipolar electrode (Plastics One, Roanoke, VA) targeted at the left medial forebrain bundle (2.8 mm posterior to bregma, 1.7 mm lateral from midsagittal suture, and 7.8 mm below dura). The electrode was secured into place using orthodontic resin. Rats received ketoprofen (5 mg/kg IP for two days) as the postoperative analgesic. ICSS training began after 7 days of recovery.
Assay of Intracranial Self-Stimulation
Apparatus
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever, colored stimulus lights above the lever, a house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via a swivel connector (Model SL2C, Plastics One, Roanoke, VA).
Behavioral Procedure
After initial shaping of lever-press responding, rats were trained under a continuous reinforcement schedule of brain stimulation using procedures similar to those described previously 31. During initial training sessions lasting 30-60 min, the house light was illuminated, and each lever press resulted in delivery of a 0.5-s train of square-wave cathodal pulses (0.1-ms pulse duration) and illumination for 0.5-s of the stimulus lights. Responses during the 0.5-s stimulation period did not earn additional stimulation. Initially, the frequency of stimulation was held constant at 126 Hz, and stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of ICSS (≥30 stimulations/min). Frequency manipulations were then introduced, and the terminal schedule consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies was presented, with a 60-s trial at each frequency. The frequency range extended from 158-56 Hz in 0.05 log increments. Each frequency trial began with a 10-s time out, during which the house light was off and responding had no scheduled consequences. During the last 5 s of this time out, 5 non-contingent “priming” stimulations were delivered once per second at the frequency available during that trial, and the stimulus lights were illuminated during each stimulation. This non-contingent stimulation was followed by a 50-s “response” phase, during which the house light was illuminated, and responding produced electrical stimulation under the continuous reinforcement schedule described above. Training continued with presentation of three sequential components per day, and intensity was again adjusted as necessary until the following three criteria were met for the last two components for at least two consecutive days: (1) ICSS rate increased as a function of brain stimulation frequency, (2) ICSS rates were ≥50% maximum control rates for at least three and no more than six of the highest brain stimulation frequencies (see Data Analysis for definition of maximum control rate), and (3) the lowest frequency to maintain ≥50 maximum control rate varied by no more than one frequency increment from the median. In general, rats were trained in groups of 10-14. The first six rats to meet training criteria were advanced to ICSS testing. As discussed previously 31,36, the remaining rats from each group were assigned to studies of lactic acid-induced stretching using methods described below.
ICSS test sessions consisted of 6 sequential components. The first component of each test session was considered to be an acclimation component, and data were discarded. Data from the second and third “baseline” components were used to calculate baseline parameters of frequency-rate curves for that session (see Data Analysis). Following these baseline components, SNC80 (1-10 mg/kg) or ARM390 (3.2-32 mg/kg) or their vehicle was administered IP as a 10 min pretreatment to 1.8% lactic acid or its vehicle (IP in a volume of 1.0 ml/kg). Three sequential test components were conducted immediately after administration of lactic acid or vehicle. Test data were included in analysis if ICSS rates during the baseline components were ≥50% maximum control rates at the highest three brain stimulation frequencies. This criterion was met for all studies reported here.
Drugs were studied using three general approaches. First, to minimize the potential for tolerance, SNC80 and ARM390 were tested once per week at 7-day intervals as a pretreatment to 1.8% lactic acid, and doses were presented in a mixed order across rats. Three-component training sessions were conducted during other weekdays. The second study was conducted to evaluate effects of more closely spaced SNC80 treatments capable of producing acute tolerance. The study was conducted over a period of two weeks. During the first week, ICSS was evaluated after SNC80 vehicle + acid vehicle and after SNC80 vehicle + 1.8% lactic acid. Test sessions were conducted on Tuesday and Friday, and the order of testing was counterbalanced. During the second week (Wednesday-Friday), a dose of 10 mg/kg SNC80 was administered on three consecutive days. On days 1 and 3, the SNC80 injection was followed by lactic acid vehicle. On day 2, the SNC80 injection was followed by 1.8% lactic acid. During both weeks, three-component training sessions were conducted on non-test weekdays. This approach was implemented for an original group of 6 rats and replicated in a second group of 3 rats. Results were similar, and data were averaged. The effects of 32 mg/kg ARM390 were also examined 24 hr after pretreatment with 10 mg/kg SNC80 in a separate group of rats. Third, SNC80 and ARM390 were tested using a schedule under which test drugs were administered twice per week (usually Tuesday and Friday), with the test drug dose being followed by 1.8% lactic acid on one test day and by lactic acid vehicle on the other test day. Doses were presented in a mixed order across rats, and training sessions were conducted on other weekdays. This last approach has been used previously in our lab to examine effects of mu and kappa opioid agonists 31,32,36.
Data Analysis
The primary dependent variable in this ICSS procedure was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these data, raw reinforcement rates from each trial in each rat were converted to Percent Maximum Control Rate (%MCR), with the maximum control rate defined as the mean of the maximal rates observed during any frequency trial of the second and third “baseline” components for that session. Thus, %MCR values for each trial were calculated as (Response Rate During a Frequency Trial ÷ Maximum Control Rate) × 100. For each test session, data from the second and third components were averaged to yield a baseline frequency-rate curve, and data from the three test components were averaged to yield a test frequency-rate curve. Baseline and test curves were then averaged across rats to yield mean baseline and test curves for each manipulation. For statistical analysis, results were compared by repeated two-way ANOVA, with treatment and ICSS frequency as the two factors. A significant ANOVA was followed by the Holm-Sidak post hoc test, and the criterion for significance was set at p<0.05.
To provide an additional summary of ICSS performance, the total number of stimulations per component obtained across all frequencies was determined, and the average number of stimulations per test component was expressed as a percentage of the average number of stimulations per baseline component during each session. These values were then averaged across rats in each experimental condition and compared by repeated ANOVA or paired T-test as appropriate. A significant one-way ANOVA was followed by the Dunnett or Newman-Keuls post hoc test, and a significant two-way ANOVA was followed by the Holm-Sidak post hoc test. The criterion for significance was set a priori at p<0.05.
Assay of Lactic Acid-Induced Stretching
Behavioral Procedure
Test sessions were conducted once per week. Test drugs were administered IP 10 min prior to treatment with 1.8% lactic acid (IP in a volume of 1 ml/kg). Immediately after acid injection, rats were placed into acrylic test chambers (31.0cm × 20.1cm × 20.0cm) for 30 min observation periods. A stretch was operationally defined as a contraction of the abdomen followed by extension of the hind limbs, and the number of stretches during the observation period was counted. SNC80 was tested at doses of 1.0-10 mg/kg, and ARM390 was administered at doses of 3.2-100 mg/kg. In addition, the effects of 10 mg/kg SNC80 were redetermined alone or after pretreatment with the delta-selective antagonist naltrindole (1.0 mg/kg IP, 20 min before SNC80) or with SNC80 itself (0.1-10 mg/kg IP, 24 hr before a second dose of 10 mg/kg SNC80). Lastly, to evaluate interactions between SNC80 and ARM390, the effects of 10 mg/kg SNC80 were determined 30 min or 24 hr after pretreatment with 32 mg/kg ARM390.
Data Analysis
The primary dependent variable was the number of stretches observed during the 30 min observation period in each rat. These data were averaged and evaluated by repeated one-way ANOVA. A significant ANOVA was followed by the Dunnett or Newman-Keuls post hoc test, and the criterion for significance was set at p<0.05.
Drugs
Lactic acid (Sigma Chemical, St. Louis, MO) was diluted in sterile water. SNC80 [(+)-4-((αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl)-N,N-diethylbenzamide] was provided in free base form (K. Rice, NIDA/NIAA, Rockvillle, MD), and a stock solution of 10 mg/ml SNC80 was prepared using 0.4% lactic acid in sterile water. Dilutions were made in sterile water. Naltrindole HCl and ARM390 HCl [N,N-diethyl-4-(phenyl-piperidin-4-ylidenemethyl)-benzamide] (also provided by K. Rice) were dissolved in sterile water. All doses are expressed in the base or salt forms described above, and all drugs were administered IP in a volume of 1 ml/kg.
RESULTS
Effects of SNC80
Figure 1 shows the antinociceptive effects of SNC80 in the assay of lactic acid-stimulated stretching. Statistical results for this and all other figures are reported in the figure legends. After pretreatment with SNC80 vehicle, IP injection of 1.8% lactic acid stimulated a pain-related stretching response. SNC80 produced a dose-dependent and naltrindole-reversible decrease in acid-stimulated stretching. In addition, 24hr pretreatment with SNC80 produced a dose-dependent acute tolerance, manifested as a significant though incomplete attenuation in the antinociceptive effects of a subsequent SNC80 injection.
Figure 1. Effects of SNC80 in the assay of lactic acid-stimulated stretching.
Abscissae: Drug treatment. Ordinates: Number of acid-stimulated stretches. (a) The left panel shows effects of SNC80 alone in a group of 6 rats (mean±SEM baseline number of stretches after vehicle treatment =18.2±1.9). One-way ANOVA revealed a significant effect of SNC80 dose [F(3,15)=22.1, p<0.0001], and asterisks (*) indicate a significant difference from vehicle treatment (Dunnett post hoc test; p<0.05). (b) The right panel shows effects of 30 min pretreatment with the delta antagonist naltrindole (NTI, 1.0 mg/kg) or 24hr pretreatment with SNC80 (0.1-10 mg/kg) on antinociception produced by 10 mg/kg SNC80 in a different group of 6 rats (control number of stretches ± SEM=24.8±2.2). One-way ANOVA revealed a significant effect of treatment [F(5,25)=28.8, p<0.0001]. The antinociceptive effect of SNC80 was blocked by naltrindole pretreatment and dose-dependently attenuated by SNC80 pretreatment. Asterisks (*) indicate significantly different from vehicle, and crosses (†) indicate significantly different from 10 SNC80 (Newman-Keuls post hoc test; p<0.05). All bars show mean±SEM.
Figure 2 shows the antinociceptive effects of SNC80 administered once per week in the assay of lactic acid-depressed ICSS. After pretreatment with SNC80 vehicle, IP injection of 1.8% lactic acid produced a pain-related depression of ICSS, manifested as a rightward shift in the ICSS frequency-rate curve (Fig. 2a) and a decrease in the total number of stimulations delivered (Fig. 2b). SNC80 had little effect on the behavioral depressant effects of lactic acid. Thus, low doses of 1.0 and 3.2 mg/kg SNC80 significantly though incompletely blocked acid-induced decreases in ICSS maintained at one stimulation frequency (100 Hz; Fig. 2c), and 1.0 mg/kg SNC80 partially though incompletely blocked the acid-induced depression in the measure of total stimulations per component (Fig. 2d). However, the high SNC80 dose of 10 mg/kg was ineffective in producing antinociception under these conditions.
Figure 2. Effects of weekly SNC80 in the assay of acid-depressed ICSS.
The top panels (a and b) show effects of SNC80 vehicle + lactic acid vehicle (Veh-Veh) and SNC80 vehicle + 1.8% lactic acid (Veh-LA). Lactic acid treatment depressed ICSS. (a) Abscissa: frequency of electrical brain stimulation in Hz (log scale). Ordinate: Percent maximum control rate (%MCR). For this group of 6 rats, the mean±SEM MCR was 60.5±2.8. Two-way ANOVA revealed main effects of frequency [F(9,45)=51.6, p<0.001] and treatment [F(1,5)=48.8, p<0.001] and a significant interaction [F(9,45)=6.8, p<0.001]. Filled symbols indicate a significant difference from Veh-Veh (Holm-Sidak post hoc test, p<0.05). (b) Abscissa: Treatment conditions. Ordinate: Percent baseline number of stimulations per component. For this group of 6 rats, the mean±SEM baseline number of stimulations per component was 289.3±30.0. The dollar sign ($) indicates significantly different from Veh-Veh (T test, t(5)=3.65, p=0.015). The bottom panels (c and d) show effects of SNC80 administered as a pretreatment to lactic acid. SNC80 partially but incompletely attenuated acid-induced depression of ICSS. (c) Abscissa and ordinate as in (a). Two-way ANOVA revealed main effects of frequency [F(9,45)=58.6, p<0.001] and treatment [F(4,20)=4.1, p=0.013] and a significant interaction [F36,180)=2.6, p<0.001], and filled symbols indicate a significant difference from Veh-LA (Holm-Sidak post hoc test, p<0.05). (d) Abscissa and ordinate as in (b). One-way ANOVA indicated a significant effect of treatment [F(5,20)=4.2, p=0.012). Dollar signs ($) indicate significantly different from Veh-Veh, and no dose of SNC80 produced an effect different from Veh-LA (Newman-Keuls, p<0.05). All points and bars show mean data, and error bars show SEM. Error bars are shown only for Veh-LA in panel c for clarity.
Figure 3 shows the effects of 10 mg/kg SNC80 administered for three consecutive days as a pretreatment to either lactic acid vehicle or 1.8% lactic acid. Data with repeated 10 mg/kg SNC80 ± acid were compared to effects of SNC80 vehicle ± acid determined the preceding week. On the first day of repeated SNC80, 10 mg/kg SNC80 was administered as a pretreatment to lactic acid vehicle, and this pretreatment produced a significant decrease in ICSS (Fig. 3a,b). On the second day, 24hr after the first SNC80 injection, 10 mg/kg SNC80 was administered as a pretreatment to lactic acid. Under these conditions of 24hr SNC80 pretreatment, the second dose of SNC80 completely blocked acid-induced depression of ICSS (Fig. 3c,d). On the third day, 10 mg/kg SNC80 was again administered as a pretreatment to lactic acid vehicle, and on this occasion, SNC80 did not significantly alter ICSS (Fig. 3a,b). Thus, SNC80 alone produced an initial reduction in ICSS on Day 1. However, acute tolerance developed to this effect, and this unmasked an antinociceptive effect of SNC80 in the assay of acid-depressed ICSS.
Figure 3. Effects of repeated daily treatment with SNC80 on control and acid-depressed ICSS.
SNC80 was administered for 3 consecutive days. On Days 1 and 3, rats received 10 mg/kg SNC80 followed by lactic acid vehicle (10 SNC80-Veh), whereas on Day 2, rats received 10 mg/kg SNC80 followed by 1.8% lactic acid (10 SNC80-LA). These results were compared to effects of SNC80 vehicle + lactic acid vehicle (Veh-Veh) and SNC80 vehicle + 1.8% lactic acid (Veh-LA). Top panels (a and b) compare effects of Veh-Veh treatment with Day 1 and Day 3 10 SNC80-Veh treatment. SNC80 decreased ICSS on Day 1 but not on Day 3. (a) Abscissa: frequency of electrical brain stimulation in Hz (log scale). Ordinate: Percent maximum control rate (%MCR). For this group of 9 rats, the mean±SEM MCR was 60.3±2.5. Two-way ANOVA revealed a significant main effect of frequency [F(9,72)=70.0, p<0.001], no effect of treatment [F(2,16)=1.8, p=0.20] and a significant interaction [F(18,144)=3.2, p<0.001], and filled symbols indicate a significant difference from Veh-Veh (Holm-Sidak post hoc test, p<0.05). (b) Abscissa: Treatment conditions. Ordinate: Percent baseline number of stimulations per component. For this group of 9 rats, the mean±SEM baseline number of stimulations per component was 281.8±25.7. One-way ANOVA indicated a significant effect of treatment [F(2,16)=4.7, p=0.025). The dollar sign ($) indicates significantly different from Veh-Veh, and the asterisk (*) indicates significantly different from Day 1 10 SNC80-Veh (Newman-Keuls, p<0.05). The bottom panels (c and d) compare effects of Veh-Veh, Veh-LA and Day 2 10 SNC80-LA. Under these conditions, SNC80 completely blocked acid-induced depression of ICSS on Day 2. (c) Abscissa and ordinate as in (a). Two-way ANOVA revealed main effects of frequency [F(9,72)=77.9, p<0.001] and treatment [F(2,16)=12.7, p<0.001] and a significant interaction [F(18,144)=3.8, p<0.001], and filled symbols indicate a significant difference from Veh-LA (Holm-Sidak post hoc test, p<0.05). (d) Abscissa and ordinate as in (b). One-way ANOVA indicated a significant effect of treatment [F(2,16)=10.0, p=0.002). The dollar sign ($) indicates significantly different from Veh-Veh, and the asterisk (*) indicates significantly different from Veh-LA (Newman-Keuls, p<0.05). All points and bars show mean±SEM.
Figure 4 shows the effects of SNC80 administered twice per week, once before lactic acid vehicle and once before 1.8% lactic acid. Under these conditions, SNC80 did not significantly alter ICSS when it was administered as a pretreatment to lactic acid vehicle. However, it produced a significant and complete blockade of acid-induced depression of ICSS.
Figure 4. Effects of twice-weekly SNC80 on control and acid-depressed ICSS.
SNC80 was administered twice each week, once before lactic acid vehicle (SNC80-Veh) and once before 1.8% lactic acid (SNC80-LA), with at least 3 days between SNC80 doses. Under these conditions, SNC80 had no effect on control ICSS, but dose-dependently blocked acid-induced depression of ICSS. (a and b) Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: Percent maximum control response rate (%MCR). In this group of 6 rats, the mean±SEM MCR was 48.0±5.0. The left panel (a) shows data for SNC80 administered as a pretreatment to lactic acid vehicle. Two-way ANOVA revealed a significant main effect of frequency [F(9,45)=58.8, p<0.001] but not of SNC80 dose [F(3,15)=0.4, p=0.74], and the interaction was also not significant [F(27,135)=0.6, p=0.96]. The center panel (b) shows data for SNC80 administered as a pretreatment to 1.8% lactic acid. Two-way ANOVA revealed main effects of frequency [F(9,45)=122.8, p<0.001] and SNC80 dose [F(3,15)=5.5, p=0.009], and a significant interaction [F(27,135)=1.6, p=0.044]. Filled symbols indicate a significant difference from Veh-LA (Holm-Sidak post hoc test, p<0.05). (c) The right panel shows SNC80 effects on the total number of stimulations per component. In this group of 6 rats, the mean±SEM baseline number of stimulations/component was 221±39.2. Abscissa: Dose SNC80 in mg/kg. Ordinate: Percent baseline number of stimulations per component. Two-way ANOVA indicated a significant main effect of acid treatment [F(1,5)=11.8, p=0.019], no significant main effect of SNC80 dose [F(3,15)=1.9, p=0.18, and a significant interaction [F(3,15)=3.3, p=0.048]. The dollar sign ($) indicates a significant effect of acid treatment at a given dose of SNC80, and the asterisk (*) indicates a significant effect of SNC80 dose on acid-induced depression of ICSS (Holm-Sidak post hoc test, p<0.05). All points and bars show mean±SEM.
Effects of ARM390
In contrast to the effects of SNC80, Figure 5 shows that ARM390 at doses up to 32 mg/kg failed to significantly alter acid-stimulated stretching. A higher dose of 100 mg/kg ARM390 was tested in three rats. Stretching was nearly eliminated in one rat, but this rat died approximately 2 hr after the session, and doses greater than 32 mg/kg were not tested further. The failure of ARM390 to significantly reduce stretching suggested that ARM390 might function as a low efficacy ligand at delta receptors capable of antagonizing effects produced by higher efficacy agonists. However, Figure 5 also shows that 30 min pretreatment with ARM390 failed to alter the antinociceptive effects of SNC80 in the assay of acid-stimulated stretching. Twenty-four hour pretreatment with 32 mg/kg ARM390 also failed to produce acute tolerance to subsequent antinociceptive effects of SNC80.
Figure 5. Effects of ARM390 in the assay of acid-stimulated stretching.
Abscissae: Drug treatment. Ordinates: Number of acid-stimulated stretches. (a) The left panel shows effects of ARM390 alone in a group of 6 rats (mean±SEM baseline number of stretches after vehicle treatment =32.5±4.4). Doses of 3.2-32 mg/kg ARM390 did not significantly alter stretching [F(3,15)=1.4, p=0.29]. A higher dose of 100 mg/kg ARM390 was tested in only 3 rats, and one of these rats died approximately 2hr after the session. (b) The right panel shows effects of 10 mg/kg SNC80 administered after pretreatment with either ARM-390 vehicle or 32 mg/kg ARM390 in 5 of the same 6 rats. ARM390 was administered either 30 min or 24 hr before SNC80. One-way ANOVA revealed a significant effect of treatment [F(3,12)=21.5, p<0.0001]. SNC80 alone significantly decreased stretching. Neither 30 min nor 24 hr pretreatment with ARM390 significantly altered SNC80 antinociception. Asterisks (*) indicate significantly different from the no treatment control (Newman-Keuls post hoc test; p<0.05). All bars show mean±SEM.
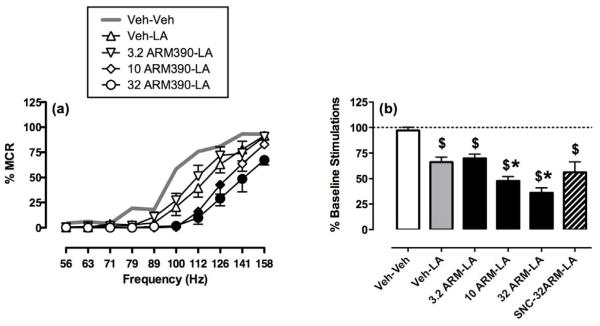
Figure 6 shows that ARM390 failed to produce antinociception in the assay of acid-depressed ICSS. When ARM390 was administered once per week (comparable to the SNC80 study shown in Fig. 2), ARM390 produced only dose-dependent exacerbation of acid-induced depression of ICSS. Twenty-four hr pretreatment with 10 mg/kg SNC80, which produced acute tolerance to ICSS rate-decreasing effects of SNC80, also produced acute tolerance to ARM390-induced exacerbation of acid-induced depression of ICSS; however, SNC80 pretreatment did not unmask an antinociceptive effect of ARM3990 (Fig. 6b, striped bar). ARM390 effects on ICSS in the absence or presence of the acid noxious stimulus were also tested under the twice-weekly dosing regimen (Figure 7, identical to the dosing regimen used for SNC80 in Fig. 4). In contrast to effects observed with SNC80 under these conditions, ARM390 failed to block acid-induced depression of ICSS. Rather, there was a trend for ARM390 to decrease both control and acid-depressed ICSS, although this trend did not achieve statistical significance in this small group of 4 rats. Overall, ARM390 failed to produce antinociception in assays of either acid-stimulated stretching or acid-depressed ICSS.
Figure 6. Effects of weekly ARM390 in the assay of acid-depressed ICSS.
Unlike SNC80, ARM390 only exacerbated acid-induced depression of ICSS. (a) Abscissa: frequency of electrical brain stimulation in Hz (log scale). Ordinate: Percent maximum control rate (%MCR). For this group of 6 rats, the mean±SEM MCR was 64.4±2.5. Two-way ANOVA revealed main effects of frequency [F(9,45)=73.7, p<0.001] and treatment [F(4,20)=26.1, p<0.001] and a significant interaction [F(36,180)=6.4, p<0.001], and filled symbols indicate a significant difference from Veh-LA (Holm-Sidak post hoc test, p<0.05). (b) Abscissa: Treatment conditions. Ordinate: Percent baseline number of stimulations per component. For this group of 6 rats, the mean±SEM baseline number of stimulations per component was 292.8±28.8. One-way ANOVA indicated a significant effect of treatment [F(5,25)=4.2, p<0.0001). The dollar sign ($) indicates significantly different from Veh-Veh, and asterisks indicate a significant difference from Veh-LA (Newman-Keuls, p<0.05). All points and bars show mean±SEM.
Figure 7. Effects of twice-weekly ARM390 on control and acid-depressed ICSS.
ARM390 was administered twice each week, once before lactic acid vehicle and once before 1.8% lactic acid, with at least 3 days between ARM390 doses. Abscissa: Dose ARM390 in mg/kg. Ordinate: Percent baseline number of stimulations per component, and for this group of four rats, the mean±SEM baseline number of stimulations per component was 276.5±33.9. Two-way ANOVA indicated a significant main effect of acid treatment [F(1,3)=18.6, p=0.023], but no significant effect of ARM390 [F(3,9)=1.7, p=0.23] and no significant interaction [F(3,9)=0.4, p=0.76]. All bars show mean±SEM.
DISCUSSION
This study examined effects of the delta agonist SNC80 and its congener ARM390 in complementary assays of pain-stimulated and pain-depressed behavior in rats. There were three main findings. First, SNC80 produced antinociception in both assays. This profile of activity is similar to that produced by clinically effective analgesics such as the mu opioid receptor agonist morphine 36 and the nonsteroidal anti-inflammatory drug ketoprofen 32, and supports further consideration of delta agonists as candidate analgesics. Second, SNC80 pretreatment attenuated SNC80 antinociception in the assay of acid-stimulated stretching but unmasked SNC80 antinociception in the assay of acid-depressed ICSS. These assay-dependent and diametrically opposite effects of SNC80 pretreatment challenge conventional interpretations of antinociceptive tolerance. Finally, the SNC80 congener ARM390 failed to produce antinociception in assays of either pain-stimulated or pain-depressed behavior. These results do not support previous reports of the antinociceptive efficacy of ARM390.
Effects of SNC80 on pain-stimulated behavior
In agreement with previous studies using other assays of pain-stimulated behavior, SNC80 produced a dose-dependent and naltrindole-reversible blockade of acid-stimulated stretching in rats. Systemic administration of SNC80 and other nonpeptidic delta agonists appears to be most effective in blocking pain-stimulated behaviors elicited by visceral chemical irritants, such as stretching/writhing elicited by intraperitoneal injection of dilute acid or abdominally directed grooming elicited by intra-bladder treatment with the capsaicin analog resiniferatoxin 12,16,41,42,44. SNC80 also reduced hypersensitive withdrawal responses from thermal or mechanical stimuli in models of cutaneous inflammation in mice, rats and rhesus monkeys 5,15,16,37. Lastly, SNC80 has been reported to attenuate withdrawal responses elicited by acute noxious thermal and mechanical stimuli, although its efficacy in assays of acute thermal and mechanical pain is weaker and less reliable than in assays of chemical or inflammatory pain 3,15,16,30. These behavioral effects of delta agonists have been related to neurochemical findings that chemical and inflammatory stimuli promote the surface expression of delta receptors on neurons, and together, these findings have been interpreted to suggest that delta agonists might be especially useful for the treatment of pain related to inflammation 43.
Twenty-four hour pretreatment with SNC80 produced a dose-dependent acute tolerance to the antinociceptive effects of a subsequent SNC80 injection in the assay of acid-stimulated stretching. This agrees with previous studies reporting that SNC80 pre-exposure produces acute tolerance to subsequent SNC80 antinociception in assays of pain-stimulated behavior in mice, as well as tolerance to other SNC80 effects including convulsant and locomotor effects in rats and suppression of food-maintained responding in rhesus monkeys 6,22,37.
Effects of SNC80 on pain-depressed behavior
This is the first study to examine effects of delta agonists on pain-depressed behavior. As reported previously, intraperitoneal injection of dilute lactic acid was sufficient as a noxious stimulus to produce a pain-related depression of ICSS in rats 31,32,36. SNC80 produced antinociception insofar as it attenuated acid-induced depression of ICSS. However, in contrast to the acute tolerance observed in the assay of acid-stimulated stretching, SNC80 pretreatment unmasked SNC80 antinociception in the assay of acid-depressed ICSS. A likely basis for this dissociation is that the net effect of any candidate analgesic on pain-stimulated and pain-depressed behavior reflects an integration of effects not only on sensory sensitivity to the noxious stimulus, but also on other facets of sensory, cognitive and/or motor function that contribute to the measured behavior. For example, drug-induced motor impairment would be expected to augment apparent antinociception in an assay of pain-stimulated behavior (where analgesia also decreases the target behavior) but oppose expression of antinociception in an assay of pain-depressed behavior (where analgesia increases the target behavior). Tolerance to these confounding motor effects would produce the results observed here, and data from the ICSS assay support this possibility for SNC80. Thus, acute treatment with 10 mg/kg SNC80 produced evidence of pain-independent motor disruption insofar as it decreased ICSS in the absence of pain (Figure 3a and b, Day 1 data). Repeated SNC80 treatment produced acute tolerance to this decrease in ICSS (Figure 3a and b, Day 3 data), also produced partial tolerance to SNC80-induced decreases in acid-stimulated stretching (Figure 1), and unmasked SNC80-induced increases in acid-depressed ICSS (Figure 3c and d). Twice weekly SNC80 administration was also sufficient to produce tolerance to SNC80-induced decreases in ICSS in the absence of pain and permit full expression of antinociceptive increases in pain-depressed ICSS. Taken together, these results are consistent with the conclusion that repeated SNC80 produced both (a) transient motor disruption that rapidly tolerated and (b) more sustained antinociceptive effects, with antinociception being especially robust in the assay of acid-depressed ICSS.
Two other points also warrant mention. First, these findings suggest caution in the interpretation of evidence regarding tolerance to drug-induced antinociception in assays of pain-stimulated behavior. Such effects may reflect tolerance to nonselective effects rather than (or in addition) to analgesic effects. Moreover, this tolerance may not extend to measures of antinociception in assays of pain-depressed behavior or to clinical measures of pain relief. Second, the present results may also be related to previous findings that tolerance develops rapidly to SNC80-induced convulsant and locomotor effects and to rate-decreasing effects in assays of food-maintained responding (all effects that might impair and/or compete with pain-stimulated behaviors) but not to antidepressant-like effects in the forced swim assay in rats 6,22. In this regard, it appears that SNC80 antinociception in the present assay of pain-depressed behavior, like SNC80-induced antidepressant effects in forced-swim procedures, is relatively resistant to tolerance.
Abuse-related effects of SNC80
Further development of SNC80 as a candidate analgesic has been checked by concern over undesirable effects, the most important of which has been proconvulsant effects. Convulsant effects of SNC80 were not systematically investigated in this study, although maximal doses tested here were below those previously reported to produce convulsions in male Sprague-Dawley rats 21,35. An additional concern with any candidate analgesic is the degree to which it might produce abuse-related effects similar to those that limit use of mu opioid analgesics. ICSS procedures like that used here to assess prodepressant effects of pain have also been widely used to assess abuse-related effects of drugs, and drug-induced facilitation of ICSS is often interpreted as a rewarding effect predictive of abuse liability 9,23,40. SNC80 failed to facilitate ICSS in the absence of pain in the present study, even after acute tolerance developed to initial rate-decreasing effects. This agrees with previous findings that SNC80 failed to facilitate ICSS in rats or maintain drug self-administration in either rats or rhesus monkeys 22,30,35, and together, these findings suggest that SNC80 has relatively low abuse liability. Moreover, these effects of SNC80 differ from the profile of effects produced by mu opioid analgesics, which facilitate ICSS acutely and produce even greater facilitation after repeated treatment 1,23,40. Overall, these findings support the conclusion that SNC80 may differ from mu opioids in its ability to block pain-related depression of behavior without producing abuse-related effects in the absence of pain.
Effects of ARM390
ARM390 is a congener of SNC80 reported to produce delta receptor-mediated antinociception in mice 37,38. ARM390 was evaluated in this study on the basis of evidence to suggest that it interacts differently than SNC80 with delta receptors. Whereas SNC80 promotes rapid delta receptor desensitization and internalization in a manner consistent with acute behavioral tolerance, ARM390 was found to produce delayed delta receptor desensitization without internalization, and this low-internalization neurochemical profile was associated with resistance to acute behavioral tolerance 26,37,38. In the present study, ARM390 produced evidence of motor disruptive effects insofar as it tended to decrease ICSS in the absence of pain and exacerbate acid-induced depression of ICSS. Relative to SNC80, the rate-decreasing effects of ARM390 appeared resistant to tolerance insofar as these effects were still evident during twice-weekly treatments (Figure 7), although ARM390 exacerbation of acid-induced depression of ICSS was sensitive to cross tolerance produced by SNC80 pretreatment. This pharmacological profile of ICSS rate-decreasing effects in rats parallels the resistance to acute ARM390 tolerance but sensitivity to SNC80 cross tolerance observed for antinociceptive effects reported previously in mouse assays of pain-stimulated behavior 37. However, ARM390 did not produce antinociception in the present study. ARM390-induced decreases in acid-stimulated stretching did not achieve statistical significance, and ARM390 failed to block acid-induced depression of ICSS even in the presence of SNC80 pretreatment-induced tolerance to the rate-decreasing effects of ARM390. The reasons for the discrepancy between previous and present results are not known, and may involve such factors as the species of experimental subject (mice vs. rats) or the type of antinociceptive test (thermal and mechanical hypersensitivity elicited by complete Freunds adjuvant vs. acid-stimulated stretching and acid-depressed ICSS). The ineffectiveness of ARM390 is unlikely to reflect inadequate dosing. In mice, SNC80 and ARM390 displayed roughly equivalent antinociceptive potency 37, and in the present study, ARM390 was tested up to doses sufficient to produce rate-decreasing effects and lethality, and more than 30 times greater than antinociceptive doses of SNC80. The results also are not consistent with low efficacy of ARM390 at delta receptors, because ARM390 failed to antagonize effects of SNC80 in the assay of acid-stimulated stretching. Rather, these results suggest that ARM390 may not alter pain sensitivity, but does produce other behaviorally disruptive effects that may present as apparent antinociception in some assays of pain-stimulated behavior.
Acknowledgments
This work was supported in part by R01-DA11460 from the National Institute on Drug Abuse and R01-NS070715 from the National Institute of Neurological Disorders and Stroke. A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICT OF INTEREST
None of the authors have professional or financial relationships that could result in conflicts of interest related to work described in this manuscript.
REFERENCES
- 1.Altarifi A, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment and rate-dependence. Behav Pharmacol. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- 4.Borsook D, Becerra L, Carlezon WA, Jr., Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther. 2001;296:939–946. [PubMed] [Google Scholar]
- 6.Brandt MR, Furness MS, Rice KC, Fischer BD, Negus SS. Studies of tolerance and dependence with the delta-opioid agonist SNC80 in rhesus monkeys responding under a schedule of food presentation. J Pharmacol Exp Ther. 2001;299:629–637. [PubMed] [Google Scholar]
- 7.Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant-like effects in Sprague-Dawley rats. Psychopharmacology (Berl) 2002;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- 8.Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- 9.Carlezon WA, Jr., Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 11.Council (National Research Council) Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- 12.Craft RM, Henley SR, Haaseth RC, Hruby VJ, Porreca F. Opioid antinociception in a rat model of visceral pain: systemic versus local drug administration. J Pharmacol Exp Ther. 1995;275:1535–1542. [PubMed] [Google Scholar]
- 13.Dharmshaktu P, Tayal V, Kalra BS. Efficacy of Antidepressants as Analgesics: A Review. J Clin Pharmacol. doi: 10.1177/0091270010394852. Epub ahead of print, March 17, 2011: http://jcp.sagepub.com/content/early/2011/2003/2016/0091270010394852. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. Br J Pharmacol. 2000;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- 17.Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27:2558–2567. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg DL. Pain/Depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med. 2010;123:675–682. doi: 10.1016/j.amjmed.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Lepine JP, Angermeyer MC, Levinson D, de Girolamo G, Iwata N, Karam A, Guimaraes Borges GL, de Graaf R, Browne MO, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Jutkiewicz EM. The Antidepressant -like Effects of Delta-Opioid Receptor Agonists. Mol Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- 21.Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- 22.Jutkiewicz EM, Kaminsky ST, Rice KC, Traynor JR, Woods JH. Differential behavioral tolerance to the delta-opioid agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther. 2005;315:414–422. doi: 10.1124/jpet.105.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- 24.Lepine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19(Suppl 1):S3–7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- 25.Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26:30–36. [PMC free article] [PubMed] [Google Scholar]
- 26.Marie N, Landemore G, Debout C, Jauzac P, Allouche S. Pharmacological characterization of AR-M1000390 at human delta opioid receptors. Life Sci. 2003;73:1691–1704. doi: 10.1016/s0024-3205(03)00489-2. [DOI] [PubMed] [Google Scholar]
- 27.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 29.Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. In: Szallasi A, editor. Methods in Molecular Biology: Analgesia. Humana Press; New York: 2010. pp. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- 31.Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. doi: 10.1124/jpet.111.186783. Epub ahead of print, November 29, 2011: http://jpet.aspetjournals.org/content/early/2011/2011/2029/jpet.2111.186783.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical Assessment of Candidate Analgesic Drugs: Recent Advances and Future Challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 34.Ossipov MH, Lai J, Vanderah T, Porecca F. The Delta Opioid Receptor Subtypes and Pain Modulation. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. Marcel Dekker; New York: 2004. pp. 297–329. [Google Scholar]
- 35.Pereira Do Carmo G, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr., Negus SS. The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv. 2002;2:392–402. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- 40.Reid LD. Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs. In: Bozarth MJ, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer; Berlin: 1987. pp. 391–420. [Google Scholar]
- 41.Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, Nagase H, Yamada M. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–279. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 42.Sora I, Li XF, Funada M, Kinsey S, Uhl GR. Visceral chemical nociception in mice lacking mu-opioid receptors: effects of morphine, SNC80 and U-50,488. Eur J Pharmacol. 1999;366:R3–5. doi: 10.1016/s0014-2999(98)00933-9. [DOI] [PubMed] [Google Scholar]
- 43.Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26(Suppl 10):S10–15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- 44.Wild KD, McCormick J, Bilsky EJ, Vanderah T, McNutt RW, Chang K-J, Porreca F. Antinociceptive actions of BW373U86 in the mouse. J Pharmacol Exp Ther. 1993;267:858–865. [PubMed] [Google Scholar]