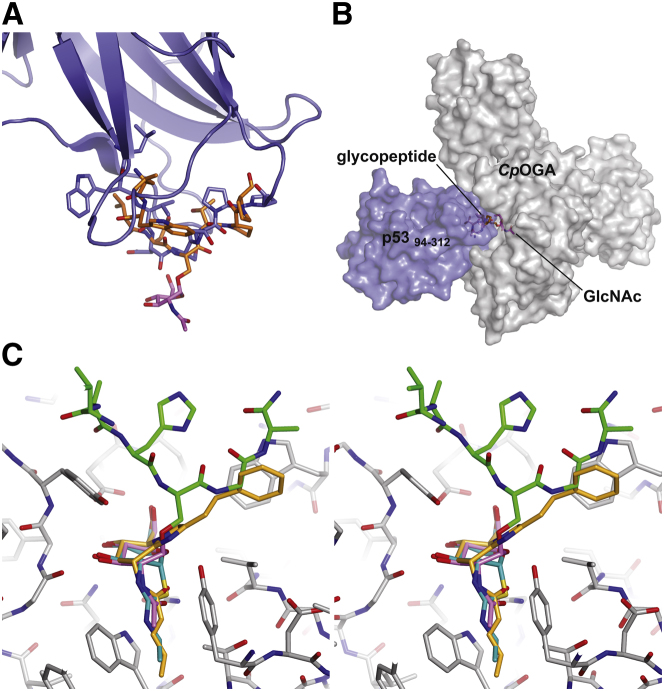
Figure 2.
Comparison of Glycopeptide Binding with the Native p53 Conformation and Inhibitor Complexes
(A) Superposition of the p53-derived glycopeptide structure as observed in the complex with CpOGA D298N (orange and pink sticks) with the corresponding region of the p53 DNA binding domain crystal structure (Cho et al., 1994) (blue sticks and cartoon; Protein Data Bank ID 1tup).
(B) Macromolecular model of CpOGA (gray) and the p53 DNA binding domain (blue) in surface representation.
(C) Comparison of substrate and inhibitor binding modes (divergent stereo image). CpOGA (gray sticks) is shown in complex with the hOGA-derived substrate glycopeptide (green and pink sticks), CpOGA-GlcNAcstatin G (Dorfmueller et al., 2010) (orange sticks), and with the thiazoline-derivative thiamet-G (blue sticks, obtained by superposition with the GH84 enzyme from B. thetaiotaomicron; Protein Data Bank ID 2vvn) (Yuzwa et al., 2008).