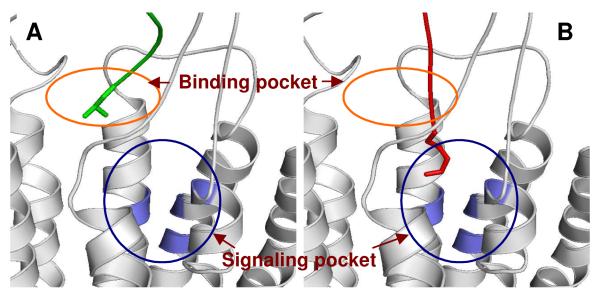
Figure 3.
Two pocket model as demonstrated by distinct binding and signaling pockets in CXCR4. As described in our earlier publication, 188 these structural models show the superimposition of the average structures obtained from computational molecular dynamics simulations of 9–CXCR4 (A) and SDF-1α–CXCR4 complexes (B). The superimposition displays the difference in the binding of the N-termini of ligands. In the case of 9 (green), the N-terminus populates site A (red circle, denoted as binding pocket), whereas in the case of SDF-1α (red), the N-terminus populates a hydrophobic region in site B (blue circle, denoted as signaling pocket) in CXCR4. The first N-terminal residue for either ligand is displayed in a stick model.