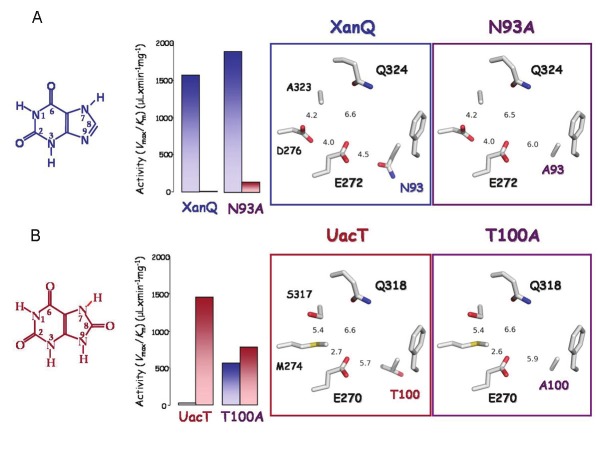
Figure 3.
Determinants of the xanthine/8-oxy-xanthine distinction between permeases XanQ and UacT. The major substrate, relative efficiencies for the uptake of xanthine (blue bars) and uric acid (red bars) and the arrangement of side chains in the presumed binding site are shown for XanQ and its mutant N93A (A) or UacT and its mutant T100A (B). The two mutants shown were the only ones leading to clear-cut changes in selectivity in a mutagenesis screen of 16 important residue positions [for a complete account of this set of mutagenesis targets, see Supplemental Figure S1]. Among the other binding-site residues, E270/272 and Q318/324 are functionally irreplaceable in either transporter, S317 contributes to the uric acid selectivity to a lesser extent and M274 has a stabilization role on the binding site integrity [26]. Transport efficiencies (Vmax/Km) for uric acid were measured at 25°C [26]. Transport efficiencies (Vmax/Km) for xanthine were measured at 25°C (A) or 37°C (B) [25,26]. The sequence of XanQ or UacT was threaded on the known x-ray structure of UraA [31] using the SWISSPROT modeling server and displayed with PyMOL v1.4 (Schrödinger, LLC). Numbers represent minimal distances in Ǻ between the indicated side chains.