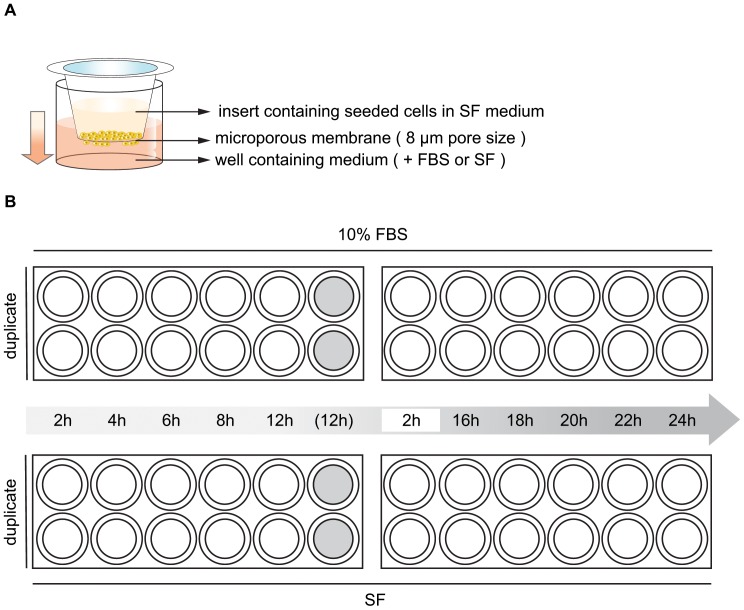
Figure 3. Conventional Transwell design for detection of time-dependent cell migration.
A. A Transwell setup consists of an upper chamber (insert) that is placed onto a lower chamber (well). The insert contains a microporous membrane (8 µm pores) allowing passage of tumor cells. After a period of serum starvation a serum-free cell suspension is seeded in the insert and exposed to medium containing potential chemoattractants (by default: medium+FBS). During incubation at 37°C and 5% CO2, cells migrate toward the bottom side of the membrane. B. Experimental design to assess time-dependent migratory behavior of cultured cells. Both migration toward FBS-containing medium and baseline migration (toward SF medium, no chemoattraction) as a negative control were included. Two times two 24-well Transwell plates were used to examine migration to FBS (positive control – top row) and baseline migration (negative control – bottom row). At ten time points during a 24-hour incubation period inserts were fixed and stained in duplicate. Two inserts containing cell-free media (grey fill) have been included throughout the experiment and fixed and stained after 12 hours incubation to assess background absorption in optical density (OD) measurements. In addition, to exclude influence of inter-plate variability on observed migration rates, each plate contained duplicate two-hour control inserts.