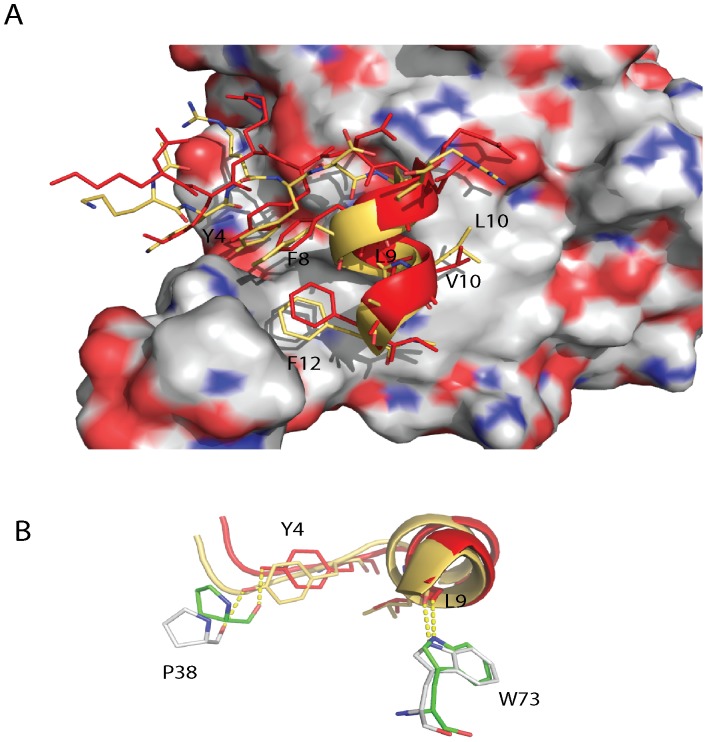
Figure 5. Amino acid changes to the C-terminal of eIF4E interacting peptides modulate their interactions with eIF4E.
An overlay of two respective snapshots from the 50 ns simulations of eIF4G1-G11A (yellow) and eIF4G1-L10V (red). The G11A mutation restrains the C-terminal conformation of the peptide allowing it to pack more efficiently and reduce the entropic cost of binding. Interestingly in the eIF4G1-L10V peptide the mutation causes the peptide to shift its orientation along the planar surface of eIF4E. The change in orientation is essentially caused by V10 trying to pack at the position vacated by the L residue. V10 is unable to pack optimally as this would result in severe disruption of the packing interaction of F8 and F12 with the surface of eIF4E. However even the suboptimal packing of V10 leads to disruption of interactions made by the F8 and F12 side chains with the result that the F12 packs differently against eIF4E and F8 is displaced. The disruption of multiple interactions and the loss of the packing of L12 against L135 and W73 significantly attenuates the interaction of the eIF4G1-L10V peptide with eIF4E. B) Two key hydrogen bonds are involved in the interface between the conserved interaction motif of the eIF4E binding peptides and the protein itself. These hydrogen bonds exist between Y4 and L9 of the conserved motif and the eIF4E surface residues P38 and W73, respectively. The hydrogen bond between Y4 and P38 is significantly displaced spatially between the eIF4E:eIF4G1-G11A (peptide in yellow, eIF4E residues in white) and eIF4E:eIF4G1-L10V (peptide in red, eIF4E residues in green)) interfaces, but the geometries of the interactions between the complexes are maintained due to the flexibility of the N-terminal tail which P38 is located on. The hydrogen bond between the carbonyl of L9 and the side chain of W73 in contrast is reasonably invariant. Deviations in the planarity of the tyrosine and phenylalanine ring systems are within the tolerances of the torsional restraints of the MD simulations. [31].