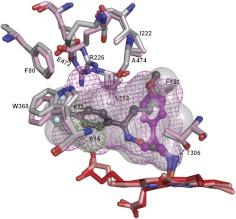
Fig. 6.
Superimposed views of FLV-bound (light gray) and TCP-bound (pink) CYP46A1 showing that the same amino acid residues undergo conformational changes upon drug binding. The active site volumes (semitransparent surface in FLV-bound and mesh in TCP-bound CYP46A1) are also shown. Two active site water molecules, 613 and 614, in TCP-bound CYP46A1 are shown as green dotted spheres. The dashed black line indicates an additional hydrogen bond between FLV-NH2 and CYP46A1. FLV is in dark gray, and TCP is in magenta. The heme groups in FLV- and TCP-bound CYP46A1 are in red and salmon, respectively. Coloring of atoms is the same as in Fig. 4.