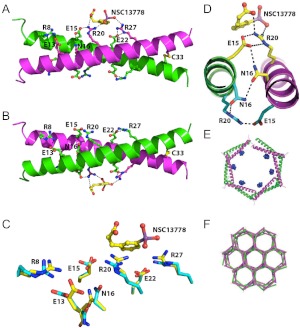
Fig. 5.
Crystal structure of the VBP leucine zipper (VBP39) in complex with NSC13778, color-coded by chain (chain A, magenta; chain B, green). A, the overall structure showing six salt bridges (three per peptide), Arg8/Glu13, Glu15/Arg20, Glu22/Arg27; asparagine bridge (Asn16); disulfide bridge (C33); and NSC13778 bound to one face of the coiled coil. B, same as A but rotated 180° along the horizontal axis. The asparagine-asparagine interaction is asymmetric. C, superposition of bound (yellow) and unbound (cyan) interfaces, showing the differences in side-chain conformations. D, interhelical N16 interaction creates a polarizing effect whereby only one conformer provides the necessary side-chain network of interactions for ligand binding (Asn16 to Glu15 to Arg20, yellow). The same set of residues on the opposing face results in an alternate network of interactions (Asn16 to Arg20 to Glu15, cyan), which precludes binding of the compound. E, the VBP leucine zippers arrange head to tail to form a hexagonal staircase, which defines the crystallographic six-fold screw axis of symmetry. The bound molecules of NSC13778 are shown in blue. F, the crystal lattice. The hexagonal coils of the leucine zippers interlock to form a continuous geometric structure that resembles a beehive honeycomb when viewed along the six-fold screw axis of symmetry. Each vertex is the interlocking interface of three adjacent hexagonal coils.