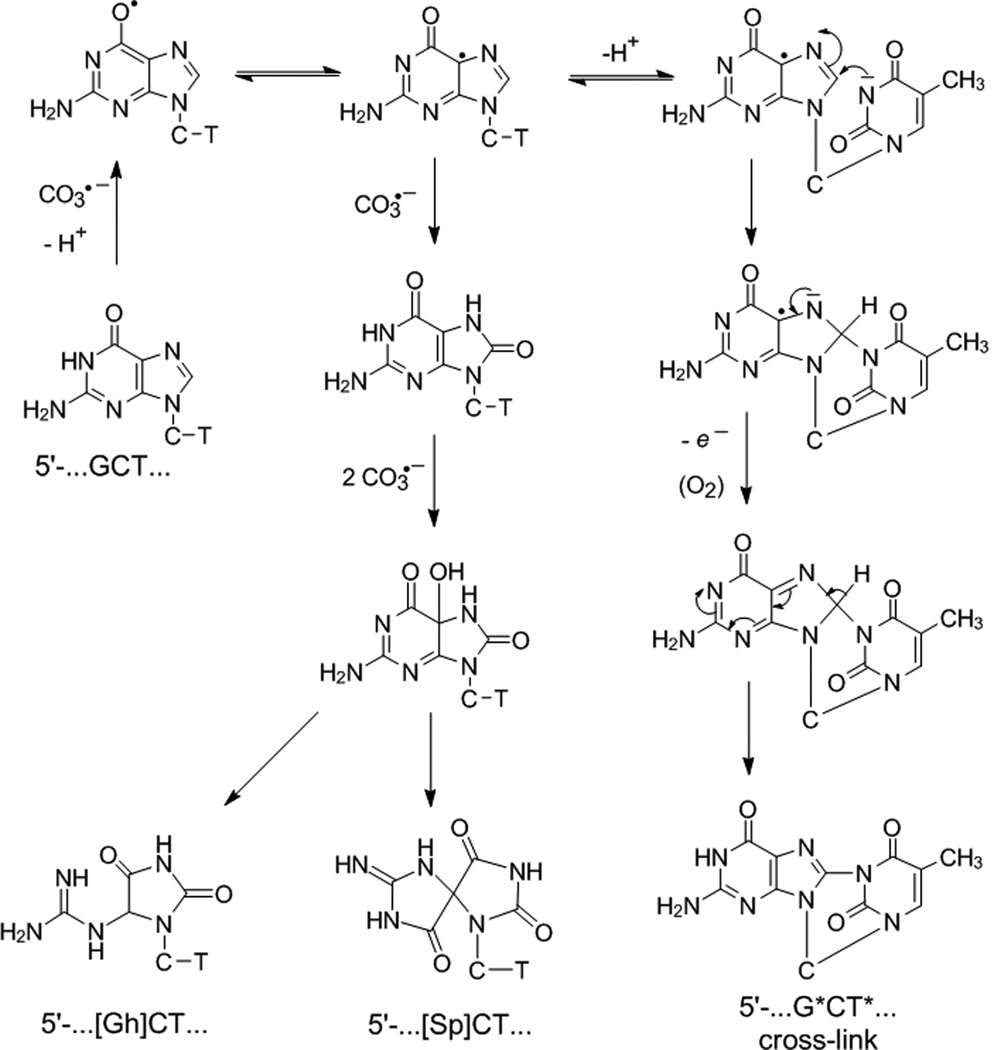
Scheme 1.
Proposed mechanism of guanine oxidation in the 5’-d(CCATCGCTACC) sequence context by carbonate radical anions. Although only the formation of the 5’-d(CCATCG*CT*ACC) product is shown, minor amounts of the isomeric 5’-d(CCAT*CG*CTACC) are also formed (Supporting Information. This Scheme is a modified version of the one published originally.[16] The main modification in Scheme involves the mechanism of deprotonation of the N3-thymine, and the resonance forms of the G(-H)• radical, which are now represented by the two conjugated resonance forms shown.[35]. The O6 and C5 radical forms of G(-H)• are expected to have lower energies than the C8-centered σ-radical protonated at N1 that we proposed earlier.[16]