Table 1.
Studies on the intramolecular Friedel-Crafts acylation.
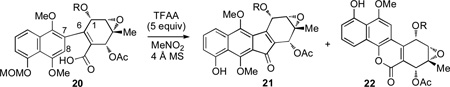 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | T [°C][a] | Selectivity [21 : 22][b] |
Yield [%][c] of 21 |
Yield [%][c] of 22 |
| 1 | 20a (R = Ac)[d] | 80 | 10 : 1 | 89 | 8 |
| 2 | 20b (R = C(O)(CH2)3N3)[d] | 23 | 4 : 1 | 58 | 15 |
| 3 | 20b [d] | 50 | >20 : 1 | 81 | - |
| 4 | 20b | 80 | >20 : 1 | 84 | - |
| 5 | 20c (R = Boc)[e] | 80 | >20 : 1 | 72[f] | - |
TFAA was added to the reaction mixture at the indicated temperature.
Selectivity was measured by crude 1H NMR analysis.
Yields are based on isolation from silica gel column chromatography.
The crude reaction mixture was further treated with TFA for complete MOM deprotection.
Treatment with pyridine[24] in EtOH was required to deprotect trifluroacetylated intermediates.
Boc group was cleaved during reactions: R = H. TFAA = trifluoroacetic anhydride, TFA = trifluoroacetic acid.
