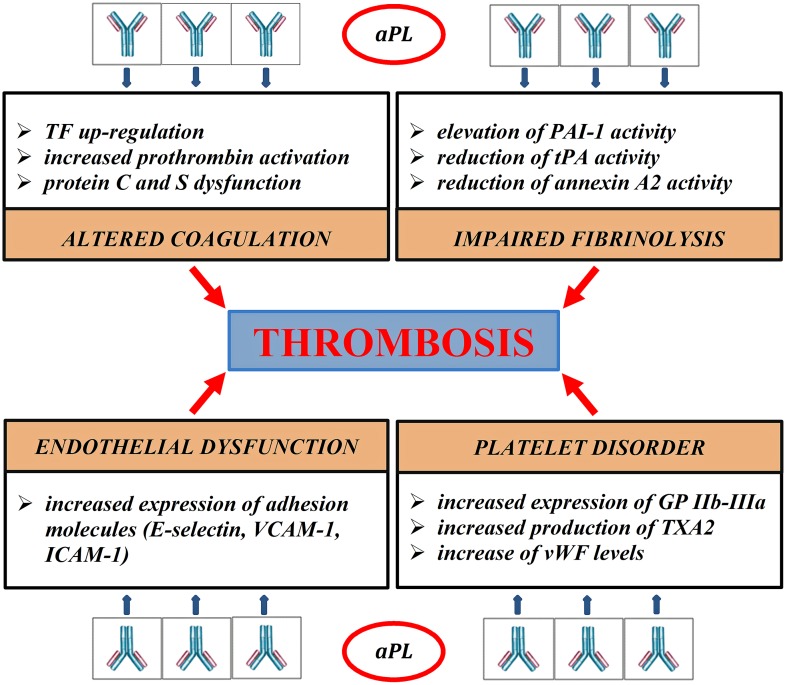
Figure 1.
Pathogenic mechanisms of thrombosis in APS. aPL disrupt the coagulation balance in many different ways. They bind directly to blood clotting-proteins, such as prothrombin, protein C, protein S, impairing their activity (Malia et al., 1990; Atsumi et al., 1998; Bertolaccini, 2012). aPL reduce the inhibitory effects of protein C on the procoagulant factors Va and VIIIa, inducing an acquired Activated Protein C Resistance (aAPCR). Protein C dysfunction also prolongs Plasminogen Activator Inhibitor-1 (PAI-1) activity, contributing to fibrinolysis disorder (Urbanus and de Laat, 2010). aPL block the tissue Plasminogen activator (tPA) activity directly and inhibit the ability of annexin 2 to potentiate tPA-mediated plasminogen activation (Krone et al., 2010). aPL stimulate the extrinsic pathway of coagulation through up-regulation of tissue factor (TF) mRNA (Lambrianides et al., 2010). APL damage platelet aggregation process. They increase platelet expression of glycoprotein IIb-IIIa (GP IIb-IIIa) and synthesis of thromboxane A2 (TXA2; Robbins et al., 1998; Pierangeli et al., 2008); aPL can also reduce the inhibitory activity of β2-GPI on von Willebrand factor (vWF), promoting platelet aggregation (Hulstein et al., 2007). In experimental models aPL generate a proinflammatory state, increasing the secretion of cytokines, such as IL1 and IL6, and promoting the expression of adhesion molecules, such as E-selectin, intracellular cell adhesion molecules 1 (ICAM1), and vascular adhesion molecules 1 (VCAM1; Pierangeli et al., 2008).