Figure 1.
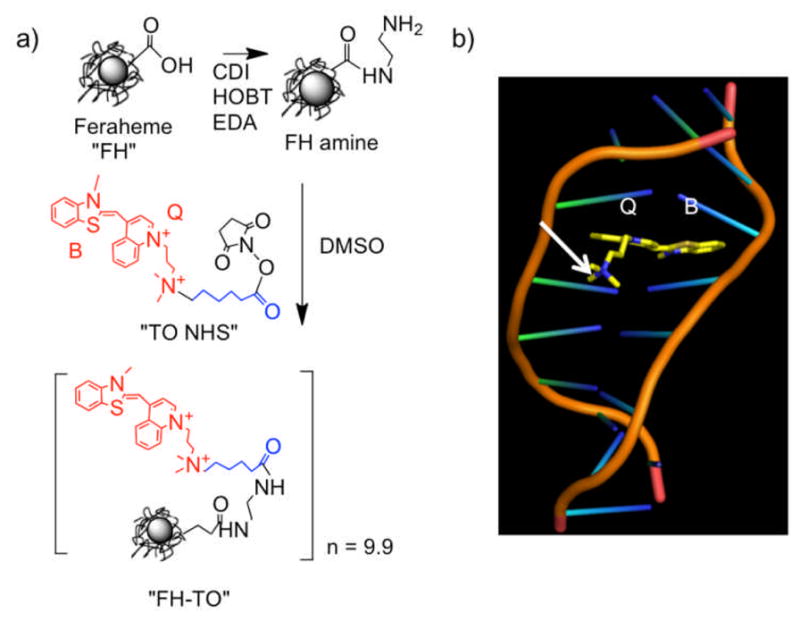
Synthesis of the FH-TO NP and TO-PRO 1 intercalation with DNA. a) An NHS ester of TO-PRO 1 (“TO NHS”) was reacted with aminated Feraheme (FH amine) to yield FH-TO with 9.9 TO’s per NP. CDI=carbodiimide, EDA=ethylene diamine, HOBT=hydroxybenzotriazole. “TO NHS” consists of TO-PRO 1 (red), a six-carbon linker (blue), and an NHS ester. b) Model showing benzathiazole (B) and quinoline (Q) rings of TO-PRO 1 (TO) intercalating into DNA. With TO NHS, a linker (not shown in (b)) maintains the quarternary positive charge (arrow) and does not interfere with the intercalation of the B and Q rings.
