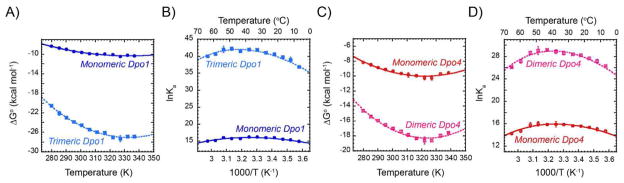
Figure 5.
Thermodynamic differences of monomeric and oligomeric Dpo1 and Dpo4 binding to DNA. Gibbs-Helmholtz plots of free energy of binding (ΔGo) for A) monomeric (solid -●-, blue) or trimeric (dashed -■-, light blue) Dpo1 and C) monomeric (solid -○-, red) or dimeric (dashed -□-, pink) Dpo4 as a function of temperature. Error bars represent the standard error from multiple experiments at each point. Lines show the fits of the data to Equation 8 giving ΔCop (cal mol−1 K−1) values of for monomeric (−0.43 ± 0.07) and trimeric (−1.45 ± 0.14) Dpo1 and monomeric (−0.68 ± 0.10) and dimeric (−1.22 ± 0.15) Dpo4. van’t Hoff plots highlighting the individual binding states for B) Dpo1 or D) Dpo4. Lines show the fits to Equation 11.