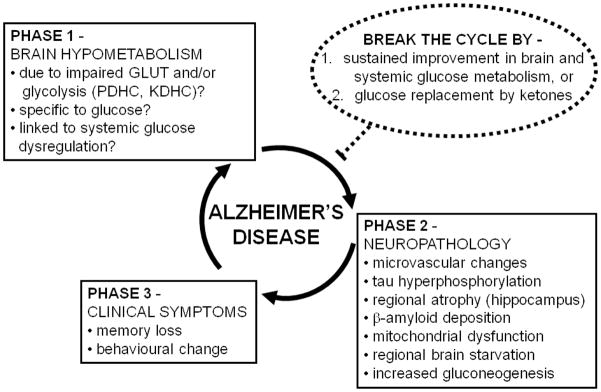
Figure 6.
Schematic overview of the concept that brain hypometabolism (Phase 1) contributes to the neuropathology underlying Alzheimer’s disease (AD; Phase 2), leading to the clinical symptoms of AD (Phase 3). The neuropathology and declining functionality of the brain can further contribute to brain hypometabolism, thereby completing a vicious cycle. In Phase 1, the hypometabolism is reportedly associated with various components of glucose utilization including one or a combination of impaired glucose transport (GLUT), impaired pyruvate dehydrogenase complex (PDHC) activity, and/or impaired α-ketoglutarate dehydrogenase complex activity (KDHC). Although the focus is on hypometabolism of glucose, it is not yet clear whether brain hypometabolism in AD affects glucose specifically or whether metabolism of other brain fuels such as ketones is also impaired. Brain hypometabolism is represented here as the first phase in the etiology of AD because it is the earliest known change in the brain associated with a risk factor predisposing to AD (presence of an apo E4 allele). In Phase 2, the microvascular changes involve altered blood-brain barrier function. In the normal brain, tau hyperphosphorylation can be stimulated by acute low glucose availability (see Section 4.1), so we propose that it is a consequence of brain hypometabolism. We propose that some brain areas are more susceptible to chronic brain hypometabolism, which can lead to regionalized brain starvation in areas that cannot adequately compensate by endogeneous gluconeogenesis. Hence, regionalized starvation and gluconeogenesis are shown here as consequences of brain hypometabolism. The cause brain hypometabolism is not yet known so it is possible that components of Phase 2 (particularly microvasculature changes and/or tau hyperphosphorylation can contribute to Phase 1, thereby further increasing the chances of developing Phase 3). Phase 3 represents the clinically observable phase which starts when the brain can no longer cope with the combination of chronic hypometabolism and neuropathological changes (Phases 1 and 2). Two related strategies are predicted to be potentially able to break the cycle (or delay it) and both involve a sustained improvement in brain fuel supply (dotted circle): (i) sustained improvement in brain glucose metabolism, which is probably dependent on sustained improvement in systemic glucose metabolism, and/or (ii) glucose replacement by ketones which are the brain’s preferred alternative physiological fuel to glucose. Such strategies are only likely to be effective if they can prevent Phase 1 becoming Phase 2, i.e. interrupting the deleterious impact of brain hypometabolism on the development of neuropathology, thereby preventing clinical symptoms of AD.