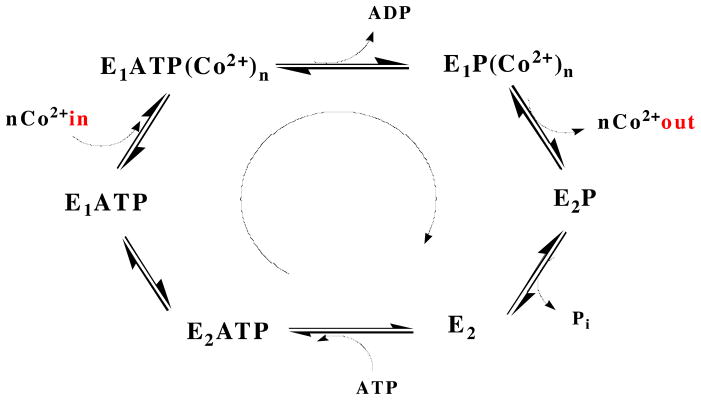
Figure 1.
The Post-Albers catalytic cycle, represented for a Co2+ ATPase. The cycle is defined by two conformational states (E1 and E2) that are interconverted by the covalent attachment and release of phosphate to and from a conserved aspartic acid residue. In the E1 state, which has high affinity for the substrate being transported, the binding site is accessible to the cytoplasm. Upon hydrolysis of ATP, a conformational change in the TM helices converts the binding site to being accessible to the periplasm (or other cellular compartment) and lowers the substrate affinity of E2. After the substrate is released, dephosphorylation occurs and the protein reverts to the E1 state.