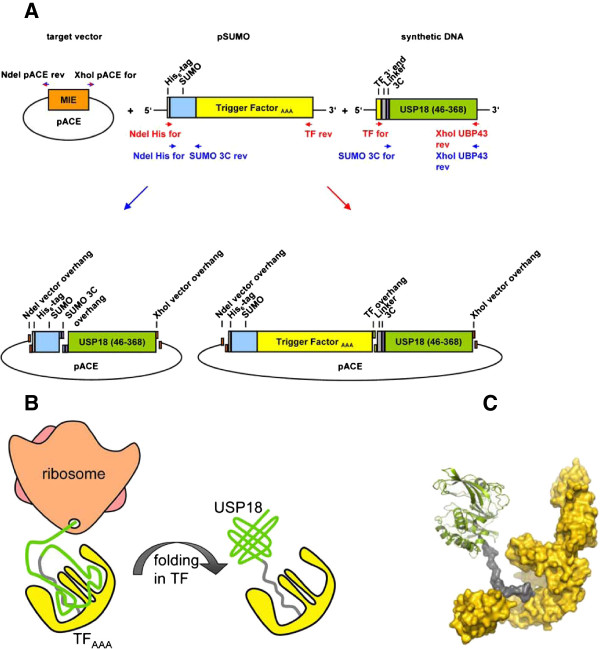
Figure 1.
(A) Generation of SUMO-USP18 and SUMO-TFAAA-USP18 expression vectors using sequence and ligation independent cloning (SLIC). The target vector pACE was linearized using primers NdeI-pACE-rev and XhoI-pACE-for. Recognition sites for the restriction enzymes NdeI and XhoI were introduced during the amplification process. Two vectors served as templates for amplification of the inserts: pSUMO encoding a His6-tag-SUMO-Trigger FactorAAA fusion protein, and a synthetic DNA construct consisting of the 3’ end of the Trigger Factor, a flexible linker, a recognition site for the 3 C protease and a USP18 cDNA for residues 46–368. For both the SUMO-USP18 and the SUMO-TFAAA-USP18 construct, two PCR products with overlapping 5’ and 3’ ends were generated. For the SUMO-USP18 construct primers NdeI-His-for together with SUMO-3C-rev as well as primers SUMO-3C-for together with XhoI-USP18-rev (blue) were used. For the SUMO-TFAAA-USP18 construct, primer NdeI-His-for was combined with TF-rev and primer TF-for with XhoI-USP18-rev (red). Treatment of the PCR products with T4 DNA polymerase in the absence of dNTPs resulted in complementary single stranded overhangs that were subsequently annealed. The resulting vectors encoded for two different USP18 fusion proteins: SUMO-USP18 and SUMO-TFAAA-USP18. Both proteins exhibit a His6-tag for purification and a SUMO-tag to enhance solubility of the protein. In the SUMO-TFAAA-USP18 protein the bacterial chaperone Trigger Factor carrying three exchanges to alanine (F43A, R44A, K45A; =TFAAA) is additionally fused to the N-terminus of USP18 to provide each expressed USP18 molecule a chaperone that facilitates folding. The flexible linker between TFAAA and USP18 was introduced to allow interaction of USP18 with the chaperone. (B) Schematic drawing of TFAAA -USP18. Newly synthesized TFAAA folds and takes up the nascent chain of USP18. The fusion protein dissociates from the ribosome and USP18 can fold in the cradle of TFAAA. (C) Molecular model of TFAAA-USP18. TFAAA is shown as surface representation and USP18 as cartoon with secondary structure elements. TFAAA is shown in yellow, the linker in grey, and USP18 in green, respectively.