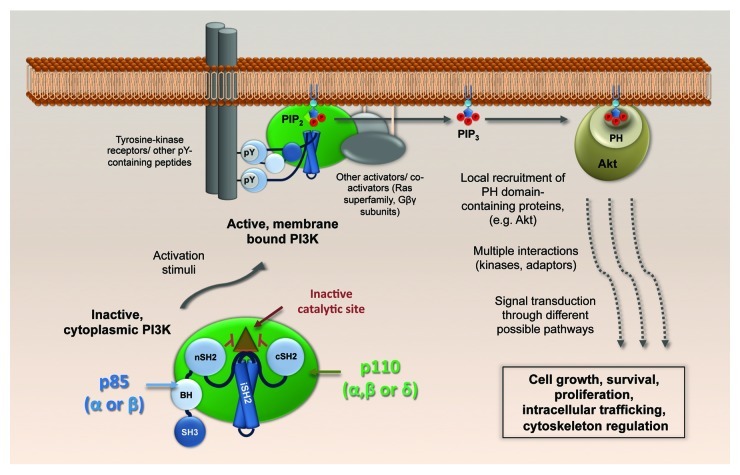
Figure 1. Physiological activation of class IA PI3K. The p110-p85 heterodimers, cytoplasmic in their basal state, dock to cellular membranes upon activation of various signal receptors, including receptor tyrosine kinases. A conformational change in p85 relieves the p110 catalytic site from inhibition by the p85 SH2 domains, and PtdIns(3,4,5)P3 is generated. Akt (and others with pleckstin homology (PH) domains) bind to PtdIns(3,4,5)P3 and build up local signaling platforms for multiple cellular pathways. SH3, Src-homology 3; BH, breakpoint-cluster region homology; nSH2, N-terminal Src-homology 2; iSH2, inter-SH2; cSH2, C-terminal Src-homology 2.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.