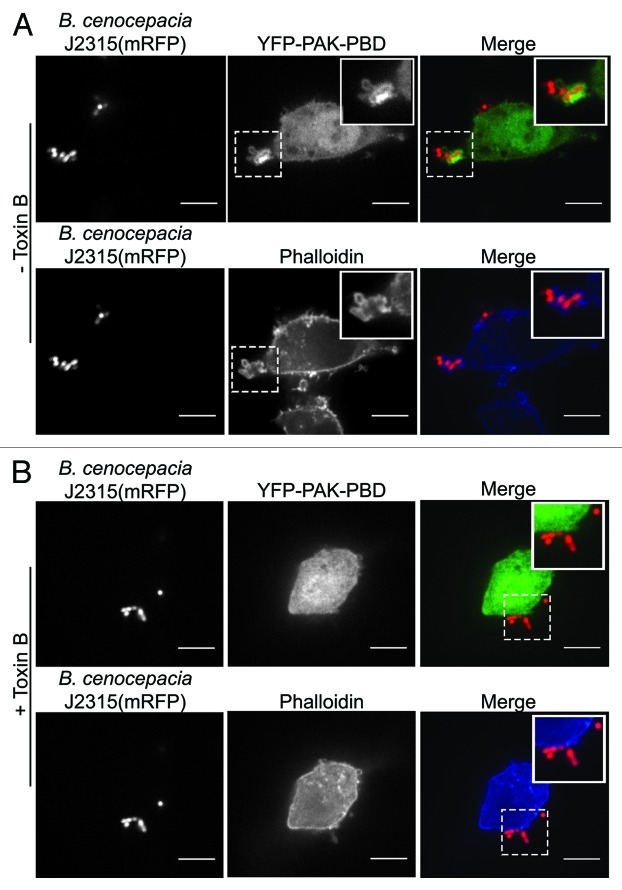
Figure 1. Recruitment of Rac/Cdc42 and F-actin to sites of B. cenocepacia entry into macrophages. To assess the recruitment of active Rac/Cdc42 to sites of B. cenocepacia phagocytosis RAW 264.7 cells were transfected with a plasmid encoding the active Rac/Cdc42 reporter p21-binding domain of p21-activated kinase (PBD-PAK) fused to yellow fluorescent protein and were infected with live B. cenocepacia J2315 expressing mRFP as previously described.7 Cells were fixed with 4% (v/v) paraformaldehyde and were permeabilized with 0.1% (v/v) Triton X-100. Cells were then stained with phalloidin conjugated to Alexafluor-647 to evaluate the organization of F-actin prior to imaging. In (A) a representative image showing the localized accumulation of YFP-PBD-PAK at a site of B. cenocepacia phagocytosis is presented (top panels). The accumulation of F-actin at the same entry site is also shown [(A), bottom panels]. The hatched boxes demarcate the region of the cells depicted in the insets. In (B) the effect of pre-treating RAW 264.7 cells expressing YFP-PAK-PBD with 100 ng/ml C. difficile toxin B for 1 h prior to infection is presented. In the top panels of part B the distribution of YFP-PAK-PBD is shown and the organization of F-actin by phalloidin staining is also demonstrated (bottom panels). Note the absence of PAK-PBD or F-actin recruitment to the site where B. cenocepacia is bound to the RAW macrophage surface. The images in (A) and (B) reflect 5 min post-infection and were acquired by spinning disk confocal microscopy. Scale bars equal 12.7 μm.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.