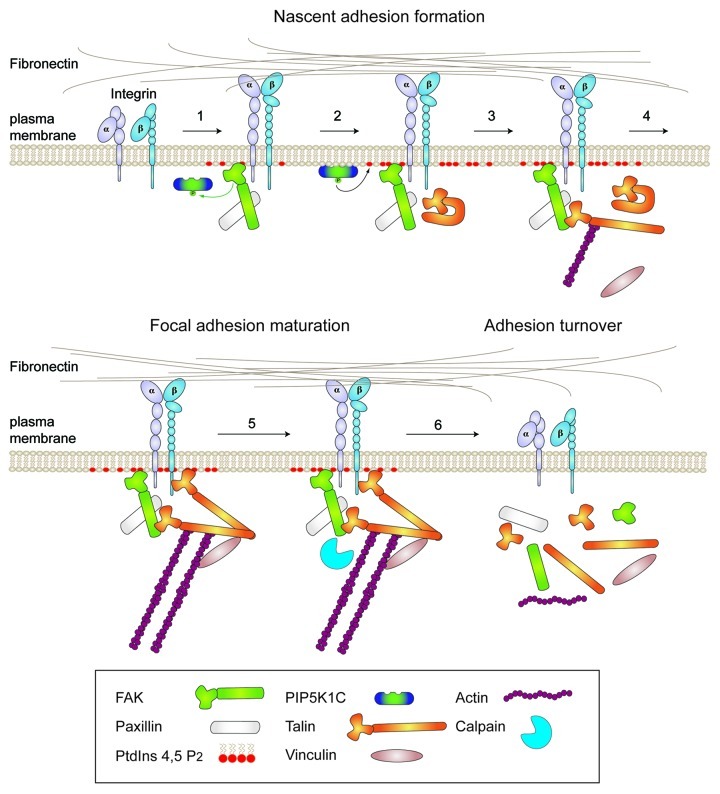
Figure 1. Model of a hierarchical linkage between integrins, FAK, talin and the control of cell motility. Simplified steps in adhesion assembly and turnover on fibronectin matrix. (1) Integrin engagement results in phosphatidylinositol-4,5-bisphosphate (PtdIns 4,5 P2) generation and the recruitment of FAK and paxillin to nascent adhesions within 15 min through undefined mechanisms. (2) FAK activation at nascent adhesions increases PtdIns 4,5 P2 generation by phosphorylating PIP5K1C. PtdIns 4,5 P2 can bind to the talin FERM domain and enable a conformational change in talin that would promote talin FERM binding to FAK and the recruitment of talin to nascent adhesions. (3) Talin dimerization allows for direct talin FERM binding to FAK and to β1-integrins for sustained adhesion signaling. (4) Myosin II-dependent tension generation and actomyosin contractility act in part through talin to enhance signaling and adhesion maturation into focal adhesions. (5) The binding of a protease such as calpain to FAK facilitates a multi-protein complex at adhesions. (6) FAK-enhanced and calpain-mediated cleavage of talin into head and rod domain fragments facilitates adhesion turnover required for efficient cell movement.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.