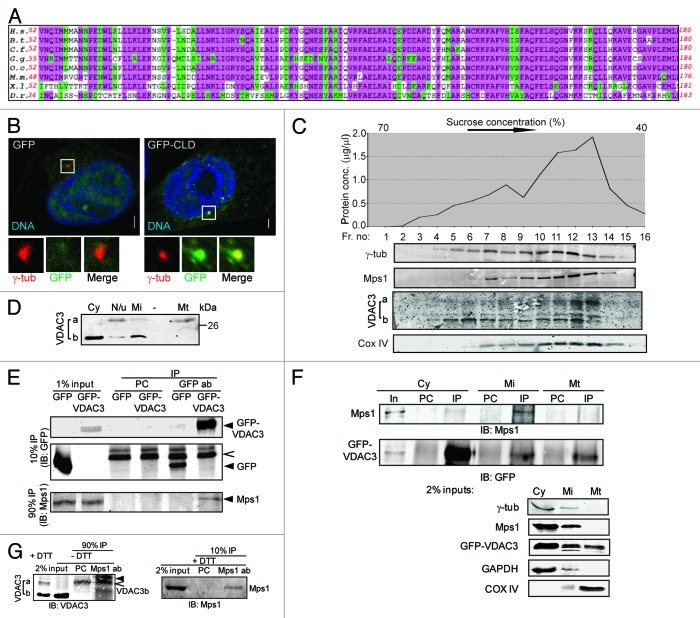
Figure 1. VDAC3 physically interacts with Mps1. (A) Conservation in the Mps1 N terminus; highlighted residues are identical (pink) or conserved in structure or function (green) in at least 75% of the species. H.s., Homo sapiens (human); B.s., Bos taurus (bovine); C.f., Canis familiaris (dog); G.g., Gallus gallus (chicken); O.c., Oryctolagus cuniculus (European rabbit); M.m., Mus musculus (mouse); X.l., Xenopus laevis (frog); D.r., Danio rerio (zebrafish). (B) S-phase arrested HeLa cells expressing GFP or GFP-Mps153-175 (GFP-CLD) were stained for γ-tubulin (γ-tub; red) and DNA (blue). Bar = 5 μm. In this and other figures, panels show 4-fold digitally magnified images of a region of interest, in this case surrounding the centrosomes. (C) Sucrose gradient fractionation of nucleui-depleted extract from HeLa cells, where the graph represents the total protein concentrations of the fractions and the immunoblots show the distribution of indicated protein in these fractions. The absence of nuclear protein contamination was verified by immunoblotting with anti-LaminB antibody (data not shown). (D) Differential distribution of VDAC3a and VDAC3b as shown by immunoblotting of subcellular fractions [cytosolic (Cy), mitochondrial (Mt), microsomal (Mi) and pellet (N/u)] obtained using Qproteome mitochondria isolation protocol. (E) After pre-clearing with beads alone (PC), anti-GFP immunoprecipitates from S-phase arrested HEK293 cells expressing GFP or GFP-VDAC3 (arrowheads) were immunoblotted with antibodies against Mps1 and GFP. Caret indicates Protein G. (F) After pre-clearing (PC), GFP-VDAC3 immunoprecipitates (IP) from the subcellular fractions from S-phase arrested HEK293 cells expressing GFP-VDAC3 were immunoblotted as in (D). Either 1% (cytosol) or 2% (other fractions) of the input (In) were analyzed with the indicated antibodies. Although a truncated form of GFP-VDAC3 was detected in lysates of cells expressing GFP-VDAC3 (see Fig. S1F), only the band of expected size (full-length GFP-VDAC3) was shown for convenience, in (E and F). (G) After pre-clearing (PC), anti-Mps1 immunoprecipitates from S-phase arrested RPE1 cells were separated in reducing (+DTT) or non-reducing (-DTT) condition and immunoblotted with indicated antibodies. Trace amount of Protein G and IgG light chain are indicated by caret and arrowhead, respectively.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.